Abstract
Purpose
Considering the importance of incorporating quality of life (QoL) construct during the health care of patients with stage 5 chronic kidney disease (CKD) on dialysis, it is necessary to have evidence on the clinimetric properties of the instruments used for its measurement. This study aimed to establish the clinimetric properties of the Kidney Disease Quality of Life Short Form 36 (KDQOL-36) scale in patients with stage 5 CKD on dialysis in Colombia.
Methods
A scale validation study was conducted using the classical test theory methodology. The statistical analysis included exploratory factor analysis (EFA) and confirmatory (CFA) techniques performed on two independent subsamples; concurrent criterion validity assessments; internal consistency using four different coefficients; test–retest reliability; and sensitivity to change using mixed model for repeated measures.
Results
The KDQOL-36 scale was applied to 506 patients with a diagnosis of stage 5 CKD on dialysis, attended in five renal units in Colombia. The EFA endorsed the three-factor structure of the scale, and the CFA showed an adequate fit of both the original and empirical models. Spearman's correlation coefficient values ≥0.50 were found between the domains of the CKD-specific core of the KDQOL-36 scale and the KDQ. Cronbach's alpha, McDonald's omega, Greatest lower bound (GLB), and Guttman's lambda coefficients were ≥0.89, indicating a high degree of consistency. A high level of concordance correlation was found between the two moments of application of the instrument, with values for Lin's concordance correlation coefficient ≥0.7. The application of the instrument after experiencing an event that could modify the quality of life showed statistically significant differences in the scores obtained.
Conclusion
The KDQOL-36 scale is an adequate instrument for measuring QoL in Colombian patients with stage 5 CKD on dialysis.
Similar content being viewed by others
Background
Chronic kidney disease (CKD) in stage 5 also called kidney failure requires kidney replacement therapy (KRT) as part of the treatment, through peritoneal dialysis (PD), hemodialysis (HD), or kidney transplant [1, 2]. During the last decade, a steady increase in the prevalence of KRT for patients with stage 5 CKD has been documented in different countries worldwide, with HD being the most widely used KRT [3,4,5].
Patients on dialysis bear a significant burden of symptoms, experienced as part of the natural course of the disease, or concerning medication, dialysis, or dietary and lifestyle modifications, which are necessary as part of the treatment [6], with an impact on the quality of life (QOL) construct [7], and clearly on health-related quality of life (HRQoL) [8, 9]. The effect of CKD on QoL has been described in numerous studies, with some of the consistently reported findings being, (I) the decreased QoL in patients with CKD compared to the general population; (II) the progressive and significant worsening of QoL in relation to the progression of CKD; and (III) the improved QoL in kidney transplant recipients compared to patients on dialysis [10,11,12,13,14,15]. The role of the HRQoL construct as a significant predictor of morbidity and mortality in patients with CKD has also been widely documented [16,17,18,19,20].
The assessment of constructs, such as QoL, which, by their nature, cannot be assessed by conventional diagnostic tests, requires the use of patient-reported outcomes (PROs) consolidated into valid and reliable instruments called patient-reported outcome measures (PROMs) [21]. For the measurement of aspects related to the health status of CKD patients, numerous generic and disease-specific PROMs are currently available [22, 23]. The quality of the results obtained through a PROM will depend on the measurement properties with which the instrument quantifies the construct of interest in the target population [24]. That is why to use an instrument, it is necessary to carry out the processes of translation and cultural adaptation [25] if it has been originally developed in another population and to have evidence on the measurement properties of the instrument in the population of interest [24]. The Kidney Disease Quality of Life short form (KDQOL-SF) of 80 items and the Kidney Disease Quality of Life short form 36 (KDQOL-36) of 36 item [26, 27] are instruments with the best evidence of adequate clinimetric properties for measuring QoL in CKD patients [28, 29]. This has prompted the translation, cross-cultural adaptation, and validation for its use in different populations around the world [30,31,32,33,34,35,36].
Although the cross-cultural adaptation of the Spanish version of the KDQOL-36 carried out in Colombian patients is available [37], there is no validation of the instrument in the Colombian population to date. Considering the importance of assessing HRQoL in CKD patients on KRT, and the lack of an adequately validated instrument for doing so in Colombia, therefore, this study aimed to establish the clinimetric properties of the KDQOL-36 instrument in Colombian patients with stage 5 CKD on dialysis.
Methods
A scale validation study was conducted from the perspective of classical test theory (CTT).
Participants: Adult patients with a diagnosis of stage 5 CKD on dialysis attended in five renal units of the Baxter Renal Care Services® network in Bogota, Colombia. Patients were recruited by non-probabilistic, sequential, and convenience sampling, applying the following inclusion criteria: (I) being 18 years of age or older; (II) being Spanish speaking; and (III) having been in Colombia for the last 10 years. Patients with cognitive or sensory alterations that prevented the adequate application of the instrument were excluded. Sample size calculation was conducted for each of the components of the scale validation process. For the analysis of the validity of the proposed content using polychoric correlation methods, a sample of no less than 250 patients is suggested [38,39,40], so a total sample of 500 patients was considered, 250 in PD and 250 in HD. For the analyses of concurrent criterion validity, internal consistency, and test–retest reliability, the sample size calculations were performed using the PASS® statistical program, assuming a significance level of 5% and a power of 80%. For the concurrent criterion validity analysis, the estimated sample size was 70 patients, considering a population correlation coefficient of 0.4 for the null hypothesis (H0) and 0.5 for the alternative hypothesis (Ha) [41, 42]. For the internal consistency analysis, a sample size of 101 patients was estimated, taking Cronbach's alpha correlation coefficient values of 0.7 for the H0 and 0.8 for the Ha [43, 44]. For the reliability analysis using the test–retest method, assuming Lin’s concordance correlation coefficient (CCC) of 0.8 for the H0 and 0.9 for the Ha [45], the estimated sample size was 100 patients. For the proposed sensitivity to change analysis using a mixed model for repeated measures (MMRM), the sample size was calculated using the GLIMMPSE® program, taking into account the non-independence of three repeated measures over time and the distribution of patients in two dialysis modalities; considering a difference of at least 2 points and 0.5 points in the standard deviation in the score obtained between the different moments of measurement [46], the estimated sample size was 351 patients [47].
Instrument: The KDQOL-36 is a 5-point Likert scale with a generic core and a CKD-specific component. The generic core is measured by the 12-item Short Form Health Survey (SF-12), which consists of 12 items conducted to the physical and mental components, whose scores are converted into mean scores of 50 and standard deviations of 10, whereby values above 50 indicate a better health status than the reference population [48]. The reliability assessment and estimation of normative values of the SF-12 among Colombian adults are available for this country [49]. The CKD-specific component has 24 kidney disease targeted items, distributed in three domains: burden of kidney disease (4 items), symptoms and problems of kidney disease (12 items), and effects of kidney disease (8 items). Item 28, which is part of the symptoms and problems domain, has two wording options depending on the dialysis modality: 28a Hemodialysis patient only “Problems with your access site?” or 28b Peritoneal dialysis patient only “problems with your catheter site?”. The pre-coded numerical values for each item are linearly transformed to a range from 0 to 100, such that for each domain higher scores indicate a better level of HRQoL [27].
Statistical analysis: The sociodemographic and clinical data of the participants were analyzed by descriptive statistics, using percentages for categorical variables; and means or medians, with the respective standard deviation (SD) or interquartile range (IQR), for continuous variables.
Content validity: It was estimated by sequentially employing the statistical techniques of exploratory factor analysis (EFA) and confirmatory factor analysis (CFA), in two independent subsamples [50, 51]. For the EFA, Bartlett's test of sphericity and the Kaiser Meyer-Olkin test (KMO) were used to check the suitability of the correlation matrix for factor analysis [52, 53]. The number of factors to be analyzed was determined using the Kaiser criterion, the percentage of total variance explained, the eigenvalue sedimentation plot analysis, and parallel analysis [54,55,56,57,58,59]. To define the factor structure, factor loadings ≥0.3 were considered [60]. To the initial orthogonal solution, orthogonal (varimax) and then oblique (promax and oblimin) rotations were performed in order to select the solution with the best clinically interpretable model. For the CFA, considering the ordinal nature of the scale items, the estimation of the models was performed from a polychoric correlation matrix, using the weighted least squares (WLS) method [61, 62]. To assess the goodness of fit of the models, measures of absolute fit and incremental fit [63] were used, with the following specified values indicating adequate fit [64,65,66]: chi-square/degrees of freedom (X2/df; values <3), root mean square error approximation index (RMSEA; values <0.08), standardized root mean square error (SRMR; values <0.08), and values >0.9 for Comparative Fit Index (CFI), Incremental Fit Index (IFI), Tucker-Lewis Index (TLI) or Non-normalized Fit Index (NNFI), and Goodness-of-Fit Index (GFI).
Concurrent criterion validity: Through the concurrent application with another scale that measures the same construct. Considering that in Colombia there is no validated instrument for the evaluation of HRQoL in patients with stage 5 CKD on dialysis, it was considered necessary to perform the translation and cross-cultural adaptation for the Colombian population of the Kidney Disease Questionnaire (KDQ), an instrument also designed to measure the construct of QoL in patients with CKD on KRT, which consists of 26 items distributed in five domains: Physical symptoms (6 items), fatigue (6 items), depression (5 items), frustration (3 items), and relationships with others (6 items), the original version of which is in English [67, 68]. The translation and cross-cultural adaptation processes were performed following the recommendations suggested by the EORTC (European Organization for Research and Treatment of Cancer) Quality of Life Group [25]. Once the two instruments were applied, the scores for each of the domains of both instruments were calculated, the Shapiro–Wilk statistical test was given to determine whether the data had a normal distribution, and then, the Spearman correlation coefficients were calculated between the scores of the CKD-specific domains of the KDQOL-36 and the scores of the domains of the KDQ.
Internal consistency: It was performed by estimating four of the suggested coefficients: Cronbach's alpha, McDonald's omega, greatest lower bound (GLB), and Guttman's lambda [69,70,71], calculated for the CKD-specific core of the scale, for each of the three domains, and through an analysis with item removal.
Reliability: Using the test–retest method, the instrument was applied at a second time 8–10 days after the first test, during which time the HRQoL construct remained stable. Lin’s CCC was used, and the dispersion of the correlation and concordance was evaluated graphically using Bland and Altman's goodness-of-fit plots [45, 72].
Sensitivity to change: We compared the scores obtained in each of the domains of the CKD-specific component of the KDQOL-36 at three different moments of application of the instrument: (I) baseline; (II) when experiencing an event that could modify the HRQoL; and (III) once the event had ended, again considering the stability of the construct. For this purpose, MMRM were used, taking into account the presence of fixed and random effects, given by the non-independence of the three moments of application of the instrument and also by the distribution of patients in two dialysis modalities, with both between-subjects and within-subjects effects.
The CFA and the calculation of Cronbach's alpha, GLB, and Guttman's lambda coefficients were performed using the R programming language, through RStudio version 1.4.1106 using the libraries lavaan, psych, paran, polycor, and semPlot [73,74,75,76,77]. The remaining analyses of the validation and descriptive statistical component were performed with the STATA 17® program.
Results
Characteristics of the participants: The total sample included 506 patients with stage 5 CKD on dialysis, 50% on HD, and 50% on PD. In the total sample, 61% of the patients were male; the median age was 57.73 years (IQR = 43.75–67.21); the characteristics of the participants in the total sample and according to dialysis modality are shown in Table 1.
Description of CKD-specific component of KDQOL-36 scores: The lowest scores, suggesting a greater compromise or decrease in quality of life, were observed in the burden of kidney disease domain with a median of 43.75 (IQR = 25–75), followed by the effects of kidney disease domain with a median of 75 (IQR = 53.12–87.5), and finally the symptoms and problems of kidney disease domain with a median of 81.25 (IQR = 68.75–91.66). Scores in each domain by dialysis modality are shown in the Supplementary material, Figure S1.
EFA: The total sample of 506 patients was divided by simple random sampling into two, “subsample 1” and “subsample 2,” each consisting of 253 patients including both dialysis modalities. EFA was performed in the first sample and CFA in the second sample. The results of Bartlett's test of sphericity (χ2 (276) = 2010.685; p = 0.000) and the KMO test (0.894) allowed us to conclude that the correlation matrix was suitable for factor analyses. Considering that, in the initial orthogonal solution, the first three factors were found to explain 93% of the variance and had eigenvalues greater than 1.0, the characteristics of the Cattell’s scree plot, and the parallel analysis with the principal factor method, the three-factor analysis was considered adequate. The factorial solution with the best clinical interpretability was the oblique rotation (promax) (Table 2). Factor one with five items that include aspects related to the perception of interference or burden of kidney disease in life; factor two with 11 items, that gather aspects related to the physical symptoms of the disease; and factor three with seven items, that include aspects related to the limitations or effects of kidney disease in daily life. Item 28 “problems with your access site?” or “problems with your catheter site?” showed the highest uniqueness value (0.96) and did not obtain an adequate factor load in any of the three domains.
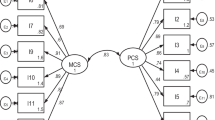
CFA: For this component, using subsample 2, the original model reported by the author [25] and the empirical model resulting from the EFA were evaluated. Figures 1 and 2 show the system of structural equations for both factor structures; the ovals represent the latent variables (domains), the squares represent the observed variables (items), the arrows in a single direction indicate the domain-item causal relationships, the arrows in two directions indicate the correlations between domains, and the arrows in dashed lines correspond to loadings that are set with a value of 1 to estimate the coefficients of the models. The goodness-of-fit estimators obtained for each of the models are presented in Table 3, with values for each of the estimators that indicate an adequate fit of both models and are very similar between them.
Concurrent criterion validity: Instruments KDQOL-36 and KDQ were applied to 199 patients, 100 patients on HD, and 99 patients on PD. Across the total sample, for each of the three domains of the CKD-specific component of the KDQOL-36, all correlations were statistically different from zero, with values obtained from Spearman's correlation coefficient that were overall >0.50. The highest correlations (ranging from 0.62 to 0.75) were found in the KDQOL-36 burden of kidney disease domain, with the KDQ scores for depression, relationships with others, and frustration; and in the KDQOL-36 symptoms and problems of kidney disease domain, with the KDQ scores for physical symptoms and fatigue; correlations that are clinically plausible and do have an adequate interpretation. Coefficients according to dialysis modality are shown in Table 4.
Internal consistency: The analysis of the CKD-specific component of the KDQOL-36 using the total sample of 506 patients resulted in similar values for the Cronbach's alpha, McDonald's omega, GLB, and Guttman's lambda coefficients, which were between 0.89 and 0.94, indicating a high level of consistency. Likewise, when the analysis was performed for each of the three domains, values for the four coefficients were identified in a range between 0.79 and 0.88. The values obtained for each coefficient in the total sample and according to dialysis modality are shown in Table 5. In the analysis with item removal using the total sample, no increase in Cronbach's alpha or Guttman's lambda coefficients was observed; however, a slight increase in the McDonald’s omega coefficient was observed when item 28 “problems with your access site?” or “problems with your catheter site?” was removed. In the analysis by KRT modality, when item 28 was removed, both in the sample of hemodialysis and peritoneal dialysis patients, a discrete increase in the level of consistency was observed for Guttman’s lambda and McDonald’s omega coefficients. The values of Cronbach's alpha, McDonald's omega, and Guttman's lambda coefficients obtained in the item removal analyses are shown in the Supplementary material, Tables S1, S2, and S3.
Test–retest reliability: The KDQOL-36 instrument was applied at a second time to 200 patients, 100 patients on HD, and 100 patients on PD, with a median of 8 days between the two assessments (IQR = 8–10). The analysis of Lin's CCC both in the total sample and by KRT modality for each of the three domains, resulted in all cases in coefficients that were statistically different from zero, with values ≥0.7 (Table 6). In the Bland and Altman plots for each of the three domains, it is evident that the average difference between the first and second application is minimal, with a high level of agreement that remains stable for the entire measurement range of the instrument, being higher for the domain effects of kidney disease (Fig. 3).
Sensitivity to change: The KDQOL-36 instrument was applied at three different times: (I) baseline; (II) when experiencing an event that could modify the quality of life; and (III) once the event was over, again considering the stability of the construct, to 351 patients, 92% in hemodialysis (n = 324 patients) and 8% in peritoneal dialysis (n = 27 patients). The scores obtained in each domain at the three time points are shown in the Supplementary material, Table S4. The analysis using MMRM resulted for each of the three domains in statistically significant differences between the scores obtained with the application of the instrument at different points in time, which shows the instrument's capacity to detect changes in the measurement of the construct as it changes. Coefficients, 95% confidence intervals, and p values obtained for each of the three domains are shown in Table 7. Supplementary material, Table S5, shows the values obtained in the pairwise comparisons by dialysis modality.
Discussion
Given the need to incorporate QOL as a health outcome during the care of patients with stage 5 CKD on dialysis [78, 79], it is necessary to have evidence of the psychometric properties of the instruments used to measure this construct in each target population [24, 25]. Likewise, it is crucial to generate evidence on the use of more advantageous, complementary, and widely recommended statistical methods to further advance and improve the quality of studies on the measurement properties of PROMs.
The sociodemographic and clinical characteristics of the study population are consistent with the data presented in the latest report published by the Colombian Fund for High-Cost Diseases on the status of CKD in Colombia [5], which additionally reports Bogota as the region with the highest estimated prevalence of KRT in the country, suggesting an adequate representativeness of the study population.
The validity of the instrument was adequate, with evidence of content and concurrent criterion validity. The EFA confirmed the factorial structure proposed in the original instrument for the CKD-specific core of the KDQOL-36, with three factors or domains regarding the burden of kidney disease, physical symptoms, and effects of the disease. For item 28 “problems with your access site?” or “problems with your catheter site?”, no adequate factor loading was found in any of the three factors, with a high uniqueness value; additionally, a discrete increase in the level of consistency was found when it was removed, suggesting that this item could be measuring an aspect other than burden, physical symptoms or effects of the disease, as part of the QoL construct. Despite using a less conservative factor loading threshold than the one used in the present validation, this same finding was evident in the validations carried out in Chinese patients [31], and in Malaysia [35], in which the loading of this item 28 on any of the three identified factors was also not reported. The CFA, performed on an independent subsample, supported the structure of the three factors or domains mentioned, with an adequate fit of the original and exploratory models, finding adequate values for each of the estimators, which were very similar between the two models. Regarding the concurrent criterion validity, despite the differences in the structure and number of items between the CKD-specific core of the KDQOL-36 and the KDQ, an adequate correlation was found between the domains of both instruments, with values of Spearman's correlation coefficient overall ≥0.50. This finding is consistent with what was reported in the validations of the instrument carried out in Arabia, Malaysia, and Ethiopia, which used evidence of different types of validity, such as convergent construct validity and discriminant construct validity [34, 36].
The instrument was reliable, showing evidence of internal consistency and test–retest reliability. In the internal consistency analysis, values for Cronbach's alpha, McDonald's omega, GLB, and Guttman's lambda coefficients were found that indicate a high level of consistency of the three CKD-specific domains of the KDQOL-36. This finding is consistent with what was reported in the studies carried out to validate the instrument in dialysis patients in Thailand [30], China [31], the United States [32], Indonesia [33], Arabia [34], Malaysia [35] and Ethiopia [36] in which Cronbach's alpha coefficient was used as the only measure of internal consistency. It is worth mentioning the discrete increase in the McDonald's omega and Guttman's lambda coefficients when item 28 “problems with your access site?” or “problems with your catheter site?” was removed. Regarding test–retest reliability, the calculation of Lin's concordance correlation coefficient for each of the kidney disease-specific domains of the KDQOL-36, and its corresponding graphical analysis of Bland and Altman limits, allowed us to confirm the stability of the measurements obtained with the instrument at two separate moments in time, considering that the construct remained stable. This finding is consistent with what was reported in validation studies of the instrument carried out in dialysis patients in Thailand [30], China [31], Arabia [34], Malaysia [35], Indonesia [33], and Ethiopia [36] in which evidence of test–retest reliability was generated, although its estimation was carried out using the intraclass correlation coefficient (ICC) or Pearson correlation coefficient. Concerning sensitivity to change, statistically significant differences were found between the scores obtained with the application of the instrument when experiencing an event that could modify the quality of life, which corroborated the instrument's capacity to detect changes in the measurements of the construct as it varies.
The validity and reliability findings of the CKD-specific core of the KDQOL-36 scale in the Colombian population using more advantageous, complementary, and widely recommended statistical methods are consistent with the data presented in validation studies conducted in other countries [30,31,32,33,34,35,36]. Additionally, we observed findings of adequate sensitivity to change of the CKD-specific core of the KDQOL-36 scale in the Colombian population, that to the best of our knowledge, at the time of this work, none of the validation studies of the CKD-specific core of the KDQOL-36 scale have included evidence of this psychometric property of the instrument.
A possible limitation of the present study is the small sample size that was possible to obtain for the evaluation of sensitivity to change in PD patients. For further studies of this instrument, we propose the evaluation of sensitivity to change in a larger sample of patients on PD. Also, it is important to evaluate the additional psychometric properties from the perspective of item response theory, such as item- and person-fit indexes, the evaluation of person and item reliability, and the analysis of the coverage of the construct spectrum with the items of the scale.
Conclusions
The findings from this study allow us to conclude that the KDQOL-36 scale is an instrument with adequate validity, reliability, and sensitivity properties to measure the construct of quality of life in Colombian patients with stage 5 chronic kidney disease on dialysis.
Availability of data and materials
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group (2013) KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 3:1–150
National Kidney Foundation (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis 39(2 Suppl 1):S1-266. PMID: 11904577
Luxardo R, Kramer A, Gonzalez-Bedat MC, Massy ZA, Jager KJ, Rosa-Diez G, Noordzij M, Collaborators (2018) The epidemiology of renal replacement therapy in two different parts of the world: the Latin American Dialysis and Transplant Registry versus the European Renal Association-European Dialysis and Transplant Association Registry. Rev Panam Salud Publica 42:e87. https://doi.org/10.26633/RPSP.2018.87
United States Renal Data System (2022) 2022 USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD. Retrieved April 17, 2023, from https://usrds-adr.niddk.nih.gov/2022
Fondo Colombiano de Enfermedades de Alto Costo, Cuenta de Alto Costo (CAC) (2022) Situación la enfermedad renal crónica, la hipertensión arterial y la diabetes mellitus en Colombia 2021. Bogotá DC. Retrieved April 17, 2023, from https://cuentadealtocosto.org/site/publicaciones/situacion-de-la-enfermedad-renal-cronica-la-hipertension-arterial-y-la-diabetes-mellitus-en-colombia-2021/
Chen TK, Knicely DH, Grams ME (2019) Chronic kidney disease diagnosis and management: a review. JAMA 322(13):1294–1304. https://doi.org/10.1001/jama.2019.14745
World Health Organization (1997) WHOQOL: measuring quality of life. WHO, Ginebra. Retrieved April 17, 2023, from https://bit.ly/317foSB
Shumaker SA, Naughton MJ (1995) The international assessment of health-related quality of life: a theoretical perspective. In: Shumaker SA, Berson RA (eds) The international assessment of health-related quality of life: theory, translation, measurement and analysis. Rapid Communications, Oxford
Naughton MJ, Shumaker SA, Anderson RT, Czajkowski SM (1996) Psychological aspects of health-related quality of life measurement: tests and scales. In: Spilker B (eds) Quality of life and pharmac economics in clinical trial, 2nd edn. Lippincott-Raven, pp 117–131
Evans RW, Manninen DL, Garrison LP Jr, Hart LG, Blagg CR, Gutman RA, Hull AR, Lowrie EG (1985) The quality of life of patients with end-stage renal disease. N Engl J Med 312(9):553–559. https://doi.org/10.1056/NEJM198502283120905
Pagels AA, Soderkvist BK, Medin C, Hylander B, Heiwe S (2012) Health-related quality of life in different stages of chronic kidney disease and at initiation of dialysis treatment. Health Qual Life Outcomes 10:71. https://doi.org/10.1186/1477-7525-10-71
Wyld MLR, Morton RL, Clayton P, Wong MG, Jardine M, Polkinghorne K, Chadban S (2019) The impact of progressive chronic kidney disease on health-related quality-of-life: a 12-year community cohort study. Qual Life Res 28(8):2081–2090. https://doi.org/10.1007/s11136-019-02173-1
Legrand K, Speyer E, Stengel B, Frimat L, Ngueyon Sime W, Massy ZA, Fouque D, Laville M, Combe C, Jacquelinet C, Durand AC, Edet S, Gentile S, Briancon S, Ayav C (2020) Perceived health and quality of life in patients with CKD, including those with kidney failure: findings from national surveys in France. Am J Kidney Dis 75(6):868–878. https://doi.org/10.1053/j.ajkd.2019.08.026
Purnell TS, Auguste P, Crews DC, Lamprea-Montealegre J, Olufade T, Greer R, Ephraim P, Sheu J, Kostecki D, Powe NR, Rabb H, Jaar B, Boulware LE (2013) Comparison of life participation activities among adults treated by hemodialysis, peritoneal dialysis, and kidney transplantation: a systematic review. Am J Kidney Dis 62(5):953–973. https://doi.org/10.1053/j.ajkd.2013.03.022
Wang Y, Hemmelder MH, Bos WJW, Snoep JD, de Vries APJ, Dekker FW, Meuleman Y (2021) Mapping health-related quality of life after kidney transplantation by group comparisons: a systematic review. Nephrol Dial Transplant 36(12):2327–2339. https://doi.org/10.1093/ndt/gfab232
DeOreo PB (1997) Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis 30(2):204–212. https://doi.org/10.1016/s0272-6386(97)90053-6
Mapes DL, Lopes AA, Satayathum S, McCullough KP, Goodkin DA, Locatelli F, Fukuhara S, Young EW, Kurokawa K, Saito A, Bommer J, Wolfe RA, Held PJ, Port FK (2003) Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int 64(1):339–349. https://doi.org/10.1046/j.1523-1755.2003.00072.x
Porter AC, Lash JP, Xie D, Pan Q, DeLuca J, Kanthety R, Kusek JW, Lora CM, Nessel L, Ricardo AC, Wright Nunes J, Fischer MJ, Investigators CS (2016) Predictors and outcomes of health-related quality of life in adults with CKD. Clin J Am Soc Nephrol 11(7):1154–1162. https://doi.org/10.2215/CJN.09990915
van Loon IN, Bots ML, Boereboom FTJ, Grooteman MPC, Blankestijn PJ, van den Dorpel MA, Nube MJ, Ter Wee PM, Verhaar MC, Hamaker ME (2017) Quality of life as indicator of poor outcome in hemodialysis: relation with mortality in different age groups. BMC Nephrol 18(1):217. https://doi.org/10.1186/s12882-017-0621-7
Hall RK, Luciano A, Pieper C, Colon-Emeric CS (2018) Association of kidney disease quality of life (KDQOL-36) with mortality and hospitalization in older adults receiving hemodialysis. BMC Nephrol 19(1):11. https://doi.org/10.1186/s12882-017-0801-5
Weldring T, Smith SM (2013) Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs). Health Serv Insights 6:61–68. https://doi.org/10.4137/HSI.S11093
Tang E, Bansal A, Novak M, Mucsi I (2017) Patient-reported outcomes in patients with chronic kidney disease and kidney transplant-part 1. Front Med (Lausanne) 4:254. https://doi.org/10.3389/fmed.2017.00254
Nair D, Wilson FP (2019) Patient-reported outcome measures for adults with kidney disease: current measures, ongoing initiatives, and future opportunities for incorporation into patient-centered kidney care. Am J Kidney Dis 74(6):791–802. https://doi.org/10.1053/j.ajkd.2019.05.025
Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, Bouter LM, de Vet HC (2010) The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: an international Delphi study. Qual Life Res 19(4):539–549. https://doi.org/10.1007/s11136-010-9606-8
Kuliś D, Bottomley A, Velikova G, Greimel E, Koller M (2017) EORTC quality of life group translation procedure, 4th edn. EORTC Quality of life unit, Brussels
Hays RD, Kallich JD, Mapes DL, Coons SJ, Amin N, Carter WB, Kamberg C (1997) Kidney disease quality of life short form (KDQOL-SF), version 1.3: a manual for use and scoring. Rand. Retrieved April 17, 2023, from https://www.rand.org/content/dam/rand/pubs/papers/2006/P7994.pdf
Hays RD, Kallich J, Mapes DL, Coons SJ, Carter WB (2000) Kidney disease and quality of life™ (KDQOL™-36), English version 1. RAND and the University of Arizona. Retrieved April 17, 2023, from https://www.rand.org/health-care/surveys_tools/kdqol.html
Aiyegbusi OL, Kyte D, Cockwell P, Marshall T, Gheorghe A, Keeley T, Slade A, Calvert M (2017) Measurement properties of patient-reported outcome measures (PROMs) used in adult patients with chronic kidney disease: a systematic review. PLoS ONE 12(6):e0179733. https://doi.org/10.1371/journal.pone.0179733
Carrillo-Algara AJ, Torres-Rodríguez GA, Leal-Moreno CS, Hernández-Zambrano SM (2018) Escalas para evaluar la calidad de vida en personas con enfermedad renal crónica avanzada: revisión integrativa. Enfermería Nefrológica 21:334–347
Thaweethamcharoen T, Srimongkol W, Noparatayaporn P, Jariyayothin P, Sukthinthai N, Aiyasanon N, Kitisriworapan P, Jantarakana K, Vasuvattakul S (2013) Validity and reliability of KDQOL-36 in Thai kidney disease patient. Value Health Reg Issues 2(1):98–102. https://doi.org/10.1016/j.vhri.2013.02.011
Chen JY, Choi EP, Wan EY, Chan AK, Tsang JP, Chan KH, Lo WK, Lui SL, Chu WL, Lam CL (2016) Validation of the disease-specific components of the kidney disease quality of life-36 (KDQOL-36) in Chinese patients undergoing maintenance dialysis. PLoS ONE 11(5):e0155188. https://doi.org/10.1371/journal.pone.0155188
Peipert JD, Bentler PM, Klicko K, Hays RD (2018) Psychometric properties of the kidney disease quality of life 36-item short-form survey (KDQOL-36) in the United States. Am J Kidney Dis 71(4):461–468. https://doi.org/10.1053/j.ajkd.2017.07.020
Supriyadi R, Rakhima F, Gondodiputro RS, Darmawan G (2019) Validity and reliability of the Indonesian version of kidney disease quality of life (KDQOL-36) questionnaire in hemodialysis patients at Hasan Sadikin Hospital, Bandung, Indonesia. Acta Med Indones 51(4):318–323. https://www.ncbi.nlm.nih.gov/pubmed/32041915. PMID: 32041915
Elamin S, Elbasher AHE, Ali SEE, Abu-Aisha H (2019) Arabic translation, adaptation, and validation of the kidney disease quality of life short-form 36. Saudi J Kidney Dis Transpl 30(6):1322–1332.https://doi.org/10.4103/1319-2442.275476
Goh KKK, Lai PSM, Lim SK (2019) Cross cultural adaptation and validation of the Malay kidney disease quality of life (KDQOL-36). BMC Nephrol 20(1). https://doi.org/10.1186/s12882-019-1397-8
Gebrie MH, Asfaw HM, Bilchut WH, Lindgren H, Wettergren L (2022) Psychometric properties of the kidney disease quality of life-36 (KDQOL-36) in Ethiopian patients undergoing hemodialysis. Health Qual Life Outcomes 20(1):24. https://doi.org/10.1186/s12955-022-01932-y
Chaves K, Duarte A, Vesga J (2013) Adaptación transcultural del cuestionario KDQOL SF 36 para evaluar calidad de vida en pacientes con enfermedad renal crónica en Colombia. Rev Med 21(2):34–42. https://doi.org/10.18359/rmed.1170
MacCallum RC, Browne MW, Sugawara HM (1996) Power analysis and determination of sample size for covariance structure modeling. Psychol Methods 1(2):130–149. https://doi.org/10.1037/1082-989X.1.2.130
Bentler PM, Yuan KH (1999) Structural equation modeling with small samples: test statistics. Multivar Behav Res 34(2):181–197. https://doi.org/10.1207/S15327906Mb340203
MacCallum RC, Widaman KF, Preacher KJ, Hong S (2001) Sample size in factor analysis: the role of model error. Multivariate Behav Res 36(4):611–637. https://doi.org/10.1207/S15327906MBR3604_06
Guenther WC (1977) Desk calculation of probabilities for the distribution of the sample correlation coefficient. Am Stat 31(1):45–48. https://doi.org/10.1080/00031305.1977.10479195
Bonett DG, Wright TA (2000) Sample size requirements for estimating pearson, kendall and spearman correlations. Psychometrika 65(1):23–28. https://doi.org/10.1007/BF02294183
Cortina JM (1993) What is coefficient alpha? an examination of theory and applications. J Appl Psychol 78:98–104. https://doi.org/10.1037/0021-9010.78.1.98
Norman GR, Streiner DL (1996) Bioestadística. Harcourt Brace, Madrid
Lin LI (1989) A concordance correlation coefficient to evaluate reproducibility. Biometrics 45(1):255–268. https://doi.org/10.2307/2532051
Alarcon JC, Bunch A, Ardila F, Zuniga E, Vesga JI, Rivera A, Sánchez R, Sanabria RM (2021) Impact of medium cut-off dialyzers on patient-reported outcomes: COREXH registry. Blood Purif 50(1):110–118. https://doi.org/10.1159/000508803
Kreidler SM, Muller KE, Grunwald G, Ringham BM, Coker-Dukowitz ZT, Sakhadeo UR, Barón AE, Glueck DH (2013) GLIMMPSE: online power computation for linear models with and without a baseline covariate. J Stat Soft 54(10):1–26. https://doi.org/10.18637/jss.v054.i10
Ware J Jr, Kosinski M, Keller SD (1996) A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care 34(3):220–233. https://doi.org/10.1097/00005650-199603000-00003
Ramirez-Velez R, Agredo-Zuniga RA, Jerez-Valderrama AM (2010) The reliability of preliminary normative values from the short form health survey (SF-12) questionnaire regarding Colombian adults. Rev Salud Publica 12(5):807–819. Retrieved April 17, 2023, from https://revistas.unal.edu.co/index.php/revsaludpublica/article/view/33328
Anderson JC, Gerbing DW (1988) Structural equation modeling in practice: a review and recommended two-step approach. Psychol Bull 103(3):411–423. https://doi.org/10.1037/0033-2909.103.3.411
Brown TA (2006) Confirmatory factor analysis for applied research. The Guilford Press, New York
Kaiser HF (1974) An index of factorial simplicity. Psychometrika 39:31–36. https://doi.org/10.1007/BF02291575
Bartlett MS (1951) The effect of standardization on a Chi-Square approximation in factor analysis. Biometrika 38(3–4):337–344. https://doi.org/10.1093/biomet/38.3-4.337
Kaiser HF (1960) The application of electronic computers to factor analysis. Educ Psychol Measur 20:141–151. https://doi.org/10.1177/001316446002000116
Merenda PF (1997) A guide to the proper use of factor analysis in the conduct and reporting of research: pitfalls to avoid. Meas Eval Couns Dev 30(3):156–164. https://doi.org/10.1080/07481756.1997.12068936
Hair, J.F., Black, W.C., Babin, B.J., & Anderson, R.E. (2010). Multivariate Data Analysis (7th ed). Pearson.
Cattell RB (1966) The scree test for the number of factors. Multivar Behav Res 1(2):245–276. https://doi.org/10.1207/s15327906mbr0102_10
Horn JL (1965) A rationale and test for the number of factors in factor analysis. Psychometrika 30:179–185. https://doi.org/10.1007/BF02289447
Kahn JH (2006) Factor analysis in counseling psychology research, training, and practice:principles, advances, and applications. Couns Psychol 34(5):684–718. https://doi.org/10.1177/0011000006286347
Pett MA, Lackey NR, Sullivan JJ (2003) Making sense of factor analysis: the use of factor analysis for instrument development in health care research. Sage Publications, Inc. https://doi.org/10.4135/9781412984898
Rigdon EE, Ferguson CE (1991) The performance of the polychoric correlation coefficient and selected fitting functions in confirmatory factor analysis with ordinal data. J Mark Res 28(4):491–497. https://doi.org/10.1177/002224379102800412
Li CH (2016) Confirmatory factor analysis with ordinal data: comparing robust maximum likelihood and diagonally weighted least squares. Behav Res Methods 48(3):936–949. https://doi.org/10.3758/s13428-015-0619-7
Mulaik SA, James LR, Van Alstine J, Bennett N, Lind S, Stilwell CD (1989) Evaluation of goodness-of-fit indices for structural equation models. Psychol Bull 105(3):430–445. https://doi.org/10.1037/0033-2909.105.3.430
Bentler PM (1990) Comparative fit indexes in structural models. Psychol Bull 107(2):238–246. https://doi.org/10.1037/0033-2909.107.2.238
Hu L, Bentler PM (1999) Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Model Multidisc J 6(1):1–55.https://doi.org/10.1080/10705519909540118
Schreiber JB (2017) Update to core reporting practices in structural equation modeling. Res Social Adm Pharm 13(3):634–643. https://doi.org/10.1016/j.sapharm.2016.06.006
Association between recombinant human erythropoietin and quality of life and exercise capacity of patients receiving haemodialysis. Canadian Erythropoietin Study Group. (1990). BMJ 300(6724):573–578. https://doi.org/10.1136/bmj.300.6724.573
Laupacis A, Muirhead N, Keown P, Wong C (1992) A disease-specific questionnaire for assessing quality of life in patients on hemodialysis. Nephron 60(3):302–306. https://doi.org/10.1159/000186769
Cronbach LJ (1951) Coefficient alpha and the internal structure of tests. Psychometrika 16:297–334. https://doi.org/10.1007/BF02310555
Woodhouse B, Jackson PH (1977) Lower bounds for the reliability of the total score on a test composed of non-homogeneous items. II: a search procedure to locate the greatest lower bound. Psychometrika 42(4):579–591. https://doi.org/10.1007/BF02295980
McDonald RP (1999) Test theory: a unified treatment, 1st edn. Psychology Press. https://doi.org/10.4324/9781410601087
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476):307–310. https://doi.org/10.1016/S0140-6736(86)90837-8
Rosseel Y (2022) lavaan: latent variable analysis. Retrieved April 17, 2023, from https://CRAN.R-project.org/package=lavaan
Revelle W (2021) psych: procedures for psychological, psychometric, and personality research. Retrieved April 17, 2023, from https://CRAN.R-project.org/package=psych
Dinno A (2018) paran: horn’s test of principal components/factors. Retrieved April 17, 2023, from https://CRAN.R-project.org/package=paran
Fox J (2022) polycor: polychoric and polyserial correlations. Retrieved April 17, 2023, from https://CRAN.R-project.org/package=polycor
Epskmap S (2022) semPlot: path diagrams and visual analysis of various SEM packages. Retrieved April 17, 2023, from https://CRAN.R-project.org/package=semPlot
Covic A, Seica A, Gusbeth-Tatomir P, Goldsmith D (2008) Hemoglobin normalization trials in chronic kidney disease: what should we learn about quality of life as an end point? J Nephrol 21(4):478–484. https://www.ncbi.nlm.nih.gov/pubmed/18651536. PMID: 18651536
Hasan LM, Shaheen DAH, El Kannishy GAH, Sayed-Ahmed NAH, Abd El Wahab AM (2021) Is health-related quality of life associated with adequacy of hemodialysis in chronic kidney disease patients? BMC Nephrol 22(1):334. https://doi.org/10.1186/s12882-021-02539-z
Acknowledgements
The authors would like to thank the health professionals who participated in the data collection. Special thanks and gratitude are also extended to the patient participants, without which this research would not have been possible.
Funding
Open Access funding provided by Colombia Consortium. This work was supported by Baxter Renal Care Services®. The funder was not involved in the study design, data collection, analysis, or interpretation of the data, writing of the manuscript, or decision to submit for publication.
Author information
Authors and Affiliations
Contributions
The study conception was performed by Ricardo Sánchez and Mauricio Sanabria. The study design was performed by Ricardo Sánchez and Martha Carolina Valderrama-Rios. Material preparation was performed by Martha Carolina Valderrama-Rios. Data collection was in charge of Mauricio Sanabria and Martha Carolina Valderrama-Rios. Data analysis and interpretation were performed by Ricardo Sánchez and Martha Carolina Valderrama-Rios. Tables and figures were prepared by Martha Carolina Valderrama-Rios. The first draft of the manuscript was written by Martha Carolina Valderrama-Rios. The critical revision of the manuscript was performed by Ricardo Sánchez and Mauricio Sanabria. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no financial or non-financial interests to declare that are relevant to the content of this manuscript.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki, and the resolution 8430 of 1993 of the Ministry of Health of Colombia, by which the scientific, technical, and administrative standards for health research are established. Approval was granted by the Ethics and Research Committees of the Baxter Renal Care Services® (Acta N°22 22-ago-2018), Hospital Universitario Nacional de Colombia (CEI-HUN-acta-2019-03), and School of Medicine, Universidad Nacional de Colombia (R-3-2020-160).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publish
Not applicable. This manuscript does not contain data from any individual person.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Valderrama-Rios, M.C., Sánchez, R. & Sanabria, M. Psychometric properties of the Kidney Disease Quality of Life short form 36 (KDQOL-36) scale for the assessment of quality of life in Colombian patients with chronic kidney disease on dialysis. Int Urol Nephrol (2024). https://doi.org/10.1007/s11255-024-03940-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11255-024-03940-x