Abstract
Purpose
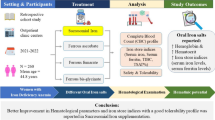
Anemia is a common complication in dialysis patients, usually treated with erythropoietin (EPO). Among available EPOs and analogs, continuous erythropoietin receptor activator (CERA) and darbepoetin alfa (DA) are the only two agents with a long duration of action, although they have almost never been formally compared in terms of efficacy. We took advantage of an accidental disruption in CERA supply to study the effect of its replacement with DA in the same patients.
Methods
The clinical and biological characteristics of 154 hemodialysis patients were retrospectively reviewed during the last 3 months on CERA compared to the first 4 months after replacement by DA, both ASE being administered by IV route. The comparison included EPO doses, hemoglobin levels, factors interfering with anemia (iron status assessment, iron doses, inflammation, quality of treatment) and was performed under the Bayesian paradigm.
Results
We found no significant differences between the two EPOs in terms of doses or hemoglobin concentrations. Factors that could potentially influence hemoglobin concentrations also did not differ under CERA or DA. The stability of hemoglobin was identical with both EPOs. We provide a conversion factor which allows comparison of cost according to local prices.
Conclusions
We conclude that, in this observational “real life” study, the two EPOs are to be considered as equivalent.
Similar content being viewed by others
Abbreviations
- ESA:
-
Erythropoiesis stimulating agent
- CERA:
-
Continuous erythropoietin receptor activator
- DA:
-
Darbepoetin alfa
- EPO:
-
Erythropoietin
- CKD:
-
Chronic kidney disease
- Hb:
-
Hemoglobin concentration
- TSC:
-
Transferrin saturation coefficient
- iPTH:
-
Immunoreactive parathyroid hormone
- CRP:
-
C-reactive protein
References
Leaf DE, Goldfarb DS (2009) Interpretation and review of health-related quality of life data in CKD patients receiving treatment for anemia. Kidney Int 75:15–24
Thorp ML, Johnson ES, Yang X, Petrik AF, Platt R, Smith DH (2009) Effect of anemia on mortality, cardiovascular hospitalizations and end-stage renal disease among patients with chronic renal disease. Nephrology 14:240–246
Donck J, Gonzales-Tabares L, Chanliau J, Martin H, Stamatelou K, Manamley N et al (2014) Preservation of anemia control and weekly ESA dosage after conversion from PEG-epoetin beta to darbepoetin alfa in adult hemodialysis patirnts: the TRANSFORM study. Adv Ther 31:1155–1168
Locatelli F, Villa G, Fransisco ALMd, Albertazzi A, Adrogue HJ, Dougherty FC et al (2007) Effect of a continuous erythropoietin receptor activator (CERA) on stable haemoglobin in patients with CKD on dialysis: once monthly administration. Curr Med Res Opin 23:969–979
Bastos K, Lucarelli LA, Francesco-Daher Ed, Filho RP, Henriquez C, Espinoza B et al (2013) CERA maintains stable hemoglobin in Latin American patients on dialysis. Int Urol Nephrol 45:1355–1364
Meier P (2010) Switch of ESA therapy from CERA to darbepoetin-alpha in chronis hemodialysis patients: a multicenter experience (absract). J Am Soc Nephrol 21:211A
Rottiers L, Wuyts P, Coppens P, Segers B, Verpooten GA, Ende Kvd (2012) Effect on haemoglobin of the switch from Mircera to Aranesp in haemodialysis (abstract). In: 41st EDTNA/ERCA international conference, Sept 2012, Strasbourg, France
Locatelli F, Covic A, Eckadt KU, Wiecek A, Vanholder R (2009) Anaemia management in patients with chronic kidney disease: a position statement by the Anemia Working Group of European Best Practice (ERBP). Nephrol Dial Transplant 24:348–354
Hughes MD (1993) Reporting Bayesian analysis of clinical trials. Stat Med 12(18):1651–1663
Dunson DB (2001) Comentary: practical advantages of Bayesian analysis of epidemiologic data. J Epidemiol 153(12):1222–1226
Ntzoufras I (2009) Bayesian modeling using WinBUGS. Hoboken, NJ
Biomédecine Ad. (2016) https://www.agence-biomedecine.fr/IMG/pdf/rapport_reinvdef.pdf. Accessed 20 Dec 2018
Rieger J, Krummel T, Petitjean P, Chantrel F, Dimitrov Y (2016) Switch of methoxy-polyethylene-glycol-epoetin beta to darbepoetin alfa in 263 dialysis patients. Ann Pharm Fr 71(1):45–48
Klinger M, Arias M, Vargemesis V, Besarab A, Sulowicz W, Gerntholtz T et al (2007) Efficacy of intravenius polyethylene glycol-epoetin beta administred every 2 weeks compared with epoetin administred 3 times weekly in patients treated by hemodialysis or peritoneal dialysis: a randomised trial. Am J Kidney Dis 50(6):989–1000
Levin NW, Fishbane S, Canedo FV, Zeig S, Nassar GM, Moran JE et al (2007) Intravenous methoxy polyethylene glycol-epoetin beta for haemoglobin control in patients with chronic kidney disease who are on dialysis: a randomised non-inferiority trial (MAXIMA). Lancet 370(9596):1415–1421
Collister D, Rigatto C, Tangri N (2017) Anemia management in chronic kidney disease and dialysis: a narrative review. Curr Opin Nephrol Hypertens 26:214–218
Allard B, Gregoire M, Ferrandini C, Ollivier C, Vrtovsnik F, Lamure E et al (2011) Etude tEmPO: interêt d'une érythropoïétine à injection mensuelle. Le Pharmacien Hospitalier 46(1):13–22
Cheung AK, Yan G, Greene T, Daugirdas JT, Dwyer JT, Levin NW et al (2002) Seasonal variations in clinical and laboratory variables among chronic hemodialysis patients. J Am Soc Nephrol 13(9):2345–2352
Kovacic V, Kovacic V (2004) Seasonal variations of clinical and biochemical properties in chronic haemodialysis. Ann Acad Med Singap 33:763–768
Funding
None.
Author information
Authors and Affiliations
Contributions
YD, JR and TH designed the study. PP, AK and FC obtained the data and were responsible for the data management. NM and TK analyzed the data. All authors contributed to drafting and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not needed for this retrospective study.
Consent to participate
All patients gave their consent to the use of the datas.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dimitrov, Y., Rieger, J., Krummel, T. et al. CERA conversion to darbepoetin alfa in 154 hemodialysis patients. Int Urol Nephrol 52, 1979–1985 (2020). https://doi.org/10.1007/s11255-020-02569-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-020-02569-w