Abstract
Introduction
Increased emergence of bacterial resistance and the limited options of novel antimicrobial agents have necessitated the reintroduction of some old antimicrobial agents. One such drug is fosfomycin, but its potential has not been explored fully, especially in India.
Aims and objectives
To analyze the in vitro activity of fosfomycin, against the urinary isolates and to compare it with in vitro activity of other orally administered antimicrobial agents.
Materials and methods
This was a prospective observational study conducted between July 2014 and June 2016. All consecutive, non-duplicate and clinically significant urinary isolates obtained from patients of all ages and both genders, diagnosed to have UTI, were included. Patients already on antibiotic therapy were excluded. Urine culture was performed by semiquantitative method on cysteine lactose electrolyte-deficient medium and the isolates obtained in significant count were subjected to antimicrobial sensitivity testing by the Kirby Bauer disk diffusion method as per CLSI guidelines.
Results
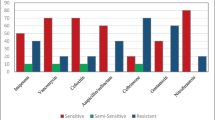
A total of 3947 non-repeating urinary isolates were included in the study, of which 2684 (68%) isolates originated from adult outpatients and remaining 1236 (32%) isolates from pediatric patients. Of these 2783 isolates were from enterobacteriaceae family. Out of these 2730 (98.1%) were sensitive to fosfomycin. Most [375 of 385 (97.4%)] Pseudomonas spp were also susceptible to fosfomycin. A majority of ESBL- (96.5%) and MBL (91.9%)-producing isolates were also susceptible to fosfomycin and so were of Gram-positive isolates [698/707 (96%)] and MRSA [61/69 (88.4%)] were susceptible to fosfomycin.
Conclusions
Fosfomycin showed an excellent in vitro activity against all urinary pathogens, including the Gram-positive or Gram-negative, ESBL and MBL producers. Fosfomycin should be considered as a highly effective alternative in treatment UTIs in both adults and pediatric patients.




Similar content being viewed by others
References
Hooton TM (2012) Clinical practice uncomplicated urinary tract infection. N Engl J Med 366:1028–1037
World Health Organization (2014) Antimicrobial resistance: Global Report on Surveillance. http://apps.who.int/iris/bitstream/10665/112642/1/9789241564748_eng.pdf. Accessed on 23 September 2016
Qiao LD, Zheng B, Chen S et al (2013) Evaluation of three-dose fosfomycin tromethamine in the treatment of patients with urinary tract infections: an uncontrolled, open-label, multicentre study. BMJ Open. doi:10.1136/bmjopen-2013-004157
Auer S, Wojna A, Hell M (2010) Oral treatment options for ambulatory patients with urinary tract infections caused by extended-spectrum-beta-lactamase-producing Escherichia coli. Antimicrob Agents Chemother 54(9):4006–4008
Falagas ME, Polemis M, Alexiou VG et al (2008) Antimicrobial resistance of Esherichia coli urinary isolates from primary care patients in Greece. Med Sci Mon 14(2):CR75–79
Boucher HW, Talbot GH, Benjamin DKJ et al (2013) Infectious Diseases Society of America. 10 × ’20 Progress development of new drugs active against Gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis 56:1685–1694
Garau J (2008) Other antimicrobials of interest in the era of extended-spectrum b-lactamases: fosfomycin, nitrofurantoin and tigecycline. Clin Microbiol Infect 14(1):198–202
Magiorakos AP, Srinivasan A, Carey RB et al (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281
Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Third Informational Supplement (2013) CLSI document M100-S23. Clinical and Laboratory Standards Institute, Wayne. http://shop.clsi.org/site/Sample_pdf/M100S23_sample.pdf. Accessed on 23 Sept 2016
Maraki S, Samonis G, Rafailidis P et al (2009) Susceptibility of urinary tract bacteria to fosfomycin. Antimicrob Agents Chemother 53:4508–4510
Cueto M, Lopez L, Hernandez JR et al (2006) In vitro activity of fosfomycin against extended-spectrum-lactamase-producing Escherichia coli and Klebsiella pneumoniae: comparison of susceptibility testing procedures. Antimicrob Agents Chemother 50:368–370
Endimiani A, Patel G, Hujer KM et al (2010) In vitro activity of fosfomycin against bla KPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 54(1):526–529
Neuner EA, Sekeres J, Hall GS et al (2012) Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 56(11):5744–5748
Rosso-Fernández C, Sojo-Dorado J, Barriga A et al (2015) Fosfomycin versus meropenem in bacteraemic urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli (FOREST): study protocol for an investigator-driven randomised controlled trial. BMJ Open. doi:10.1136/bmjopen-2014-007363
Lai B, Zheng B, Li Y, et al (2014) In vitro susceptibility of Escherichia coli strains isolated from urine samples obtained in mainland China to fosfomycin trometamol and other antibiotics: a 9-year surveillance study (2004–2012). BMC Infect Dis. doi:10.1186/1471-2334-14-66
Lu PL, Liu YC, Toh HS et al (2012) Epidemiology and antimicrobial susceptibility profiles of Gram-negative bacteria causing urinary tract infections in the Asia-Pacific region: 2009–2010 results from the study for monitoring antimicrobial resistance trends (SMART). Int J Antimicrob Agents 40(Suppl):S37–S43
Liu HY, Lin HC, Lin YC et al (2011) Antimicrobial susceptibilities of urinary extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae to fosfomycin and nitrofurantoin in a teaching hospital in Taiwan. J Microbiol Immunol Infect 44(5):364–368
Cho YH, Jung SJ, Chung HS et al (2015) Antimicrobial susceptibilities of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in health care-associated urinary tract infection: focus on susceptibility to fosfomycin. Int Urol Nephrol 47:1059–1066
de Cueto M, Hernandez JR, Lopez-Cerero L et al (2006) Activity of fosfomycin against extended-spectrum beta-lactamase producing Escherichia coli and Klebsiella pneumoniae. Enferm Infecc Microbiol Clin 24:613–616
Falagas ME, Kastoris AC, Kapaskelis AM, Karageorgopoulos DE (2010) Fosfomycin for the treatment of multidrug-resistant, including extendedspectrum beta-lactamase producing, Enterobacteriaceae infections: a systematic review. Lancet Infect Dis. doi:10.1016/S1473-3099(09)70325-1
Senol S, Tasbakan M, Pullukcu H et al (2010) Carbapenem versus fosfomycin tromethanol in the treatment of extended-spectrum beta-lactamase-producing Escherichia coli-related complicated lower urinary tract infection. J Chemother 22:355–357
Sabharwal ER, Sharma R (2015) Fosfomycin: an alternative therapy for the treatment of UTI amidst escalating antimicrobial resistance. J Clin Diagn Res 9(12):06–09
Linsenmeyer K, Strymish J, Weir S et al (2015) Activity of fosfomycin against extended-spectrum-β-lactamase-producing uropathogens in patients in the community and hospitalized patients. Antimicrob Agents Chemother. doi:10.1128/AAC.02614-15
Karlowsky JA, Denisuik AJ, Lagacé-Wiens PRS et al (2014) In vitro activity of fosfomycin against Escherichia coli isolated from patients with urinary tract infections in Canada as part of the CANWARD surveillance study. Antimicrob Agents Chemother 58(2):1252–1256
Grabein B, Graninger W, Rodríguez J, et al (2016) Intravenous fosfomycin back to the future. Systematic review and meta-analysis of the clinical literature. Clin Microbiol Infect. doi:10.1016/j.cmi.2016.12.005
Los-Arcos I, Pigrau C, Rodríguez-Pardo D et al (2016) Long-term fosfomycin-tromethamine oral therapy for difficult-to-treat chronic bacterial prostatitis. Antimicrob Agents Chemother 60:1854–1858. doi:10.1128/AAC.02611-1
Sahni RD, Balaji V, Varghese R et al (2013) Evaluation of fosfomycin activity against uropathogens in a fosfomycin-naive population in South India: a prospective study. Future Microbiol 8(5):675–680
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional guidelines for the participants were followed. It was a purely observational study. This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Patwardhan, V., Singh, S. Fosfomycin for the treatment of drug-resistant urinary tract infections: potential of an old drug not explored fully. Int Urol Nephrol 49, 1637–1643 (2017). https://doi.org/10.1007/s11255-017-1627-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-017-1627-6