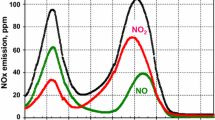
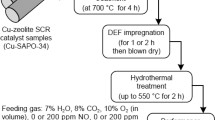
Abstract
Notwithstanding the high efficiency of the modern NH3-SCR technology in reducing NOx from Diesel engines exhausts, their removal is still a concern in view of the forthcoming application of more stringent environmental guidelines. Actually, a large fraction of the total NOx emitted by the engine derives from transient engine operations, especially at low temperature. In this context, herein we systematically investigate the effect of oxygen feed content variation under Standard SCR reaction conditions over a reference Cu-CHA catalyst. Transient Response Methods (TRM) have been applied to mimic lean to rich and rich to lean transients. Steady state experiments reveal that the higher the O2 feed content, the higher the NOx conversion and N2O formation, with N2O turning out to be very sensitive to oxygen concentration changes. Additionally, through TRM runs, it is possible to obtain quantitative information on the average fraction of oxidized Cu sites (CuII/Cutot) at the Standard SCR steady state. Particularly, the NOx conversion and the CuII fraction increase both with temperature and with O2 feed content. Hence, the higher the oxidized copper fraction, the higher the N2O formation in the Standard SCR reaction.








Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Seykens X, Kupper F, Mentink P, Ramesh S (2018) Towards ultra-low NOx emissions within GHG phase 2 constraints: main challenges and technology directions. SAE Technical Paper 2018-01-0331. https://doi.org/10.4271/2018-01-0331
Nova I, Tronconi E (2014) Urea-SCR technology for deNOx aftertreatment of diesel exhausts. Springer-Verlag, New York
Selleri T, Melas AD, Joshi A, Manara D, Perujo A, Suarez-Bertoa R (2021) An overview of lean exhaust deNOx aftertreatment technologies and NOx emission regulations in the european union. Catalysts 11:404
Brandenberger S, Kröcher O, Tissler A, Althoff R (2008) The state of the art in selective catalytic reduction of NOx by ammonia using metal-exchanged zeolite catalysts. Catal Rev: Sci Eng 50:492–531
Gramigni F, Iacobone U, Nasello ND, Selleri T, Usberi N, Nova I (2021) Review of hydrocarbon poisoning and deactivation effects on Cu-zeolite, Fe-zeolite, and vanadium-based selective catalytic reduction catalysts for NOx removal from lean exhausts. Ind Eng Chem Res 60:6403–6420
Beale AM, Gao F, Lezcano-Gonzalez I, Peden CHF, Szanyi J (2015) Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem Soc Rev 44:7371–7405
Gao F, Peden CHF (2018) Recent progress in atomic-level understanding of Cu/SSZ-13 selective catalytic reduction catalysts. Catalysts 8:140–163
Borfecchia E, Beato P, Svelle S, Olsbye U, Lamberti C, Bordiga S (2018) Cu-CHA—a model system for applied selective redox catalysis. Chem Soc Rev 47:8097–8133
Paolucci C, Di Iorio JR, Schneider WF, Gounder R (2020) Solvation and mobilization of copper active sites in zeolites by ammonia: consequences for the catalytic reduction of nitrogen cxides. Acc Chem Res 53:1881–1892
Liu C, Kubota H, Amada T, Kenichi K, Takashi T, Zen M, Kakuya U, Junya O, Atsushi S, Takuya T, Nao T, Tsuneji S, Ken-ichi S (2020) In situ spectroscopic studies on the redox cycle of NH3–SCR over Cu-CHA zeolites. ChemCatChem 12:3050–3059
Hu W, Selleri T, Gramigni F, Fenes E, Rout KR, Liu S, Nova I, Chen D, Gao X, Tronconi E (2021) On the redox mechanism of low-temperature NH3-SCR over Cu-CHA: a combined experimental and theoretical study of the eeduction half cycle. Angew Chemie 133:7273–7280
Chen L, Janssens TVW, Vennestrøm PNR, Jansson J, Skoglundh M, Gröbeck H (2020) A complete multi-site reaction mechanism for low-temperature NH3-SCR over Cu-CHA. ACS Catal 10:5646–5656
Hu W, Gramigni F, Nasello ND, Usberti N, Iacobone U, Liu S, Nova I, Gao X, Tronconi E (2022) Dynamic binuclear CuII sites in the reduction half-cycle of low-temperature NH3-SCR over Cu-CHA catalysts. ACS Catal 12(9):5263–5274
Gramigni F, Nasello ND, Usberti N, Iacobone U, Selleri T, Hu W, Liu S, Gao X, Nova I, Tronconi E (2021) Transient kinetic analysis of low-temperature NH3-SCR over Cu-CHA catalysts reveals a quadratic dependence of Cu reduction rates on CuII. ACS Catal 11:4821–4831
Gao F, Szanyi J (2018) On the hydrothermal stability of Cu/SSZ-13 SCR catalysts. Appl Catal A Gen 560:185–194
Luo J, Gao F, Kamasamudram K, Currier N, Peden CHF, Yezerets A (2017) New insights into Cu/SSZ-13 SCR catalyst acidity. Part I: nature of acidic sites probed by NH3 titration. J Catal 348:291–299
Paolucci C, Parekh AA, Khurana I, Di Iorio JR, Li H, Caballero JDA, Shih AJ, Anggara T, Delgass WN, Miller JT, Ribeiro FH, Gounder R, Schneider W (2016) Catalysis in a cage: condition-dependent speciation and dynamics of exchanged cu cations in SSZ-13 zeolites. J Am Chem Soc 138:6028–6048
Villamaina R, Liu S, Nova I, Tronconi E, Ruggeri MP, Collier J, York A, Thompsett D (2019) Speciation of Cu cations in Cu-CHA catalysts for NH3-SCR: Effects of SiO2/AlO3 ratio and Cu-loading investigated by transient response methods. ACS Catal 9:8916–8927
Chen S, Yanakiev O (2005) Transient NOx emission reduction using exhaust oxygen concentration based control for a diesel engine. SAE Technical Paper 2005-01-0372. https://doi.org/10.4271/2005-01-0372
Liu B, Yao D, Wu F, Wei L, Li X, Wang X (2019) Experimental investigation on N2O formation during the selective catalytic reduction of NOx with NH3 over Cu-SSZ-13. Ind Eng Chem Res 58:20516–20527
Zhang D, Yang RT (2018) N2O formation pathways over zeolite-supported Cu and Fe catalysts in NH3-SCR. Energy Fuels 32:2170–2182
Ahari H, Smith M, Zammit M, Price K, Jacques J, Pauly T, Wang L (2015) Impact of SCR integration on N2O emissions in diesel application. SAE Int J Passeng Cars-Mech Syst 8:526–530
Deka DJ, Daya R, Ladshaw A, Joshi S, Partridge WP (2021) A transient-response methodology based on experiments and modeling for Cu-redox half-cycle kinetic analysis on a Cu-SSZ-13 SCR catalyst. Chem Eng J 435:134219
Daya R, Trandal D, Menon U, Deka DJ, Partridge WP, Joshi S (2022) Kinetic model for the reduction of CuII sites by NO+NH3 and reoxidation of NH3-solvated CuI sites by O2 and NO in Cu-SSZ-13. ACS Catal 12:6418–6433
Grossale A, Nova I, Tronconi E (2009) Ammonia blocking of the “Fast SCR” reactivity over a commercial Fe-zeolite catalyst for diesel exhaust aftertreatment. J Catal 265:141–147
Colombo M, Nova I, Tronconi E (2010) A comparative study of the NH3-SCR reactions over a Cu-zeolite and a Fe-zeolite catalyst. Catal Today 151:223–230
Usberti N, Gramigni F, Nasello ND, Iacobone U, Selleri T, Hu W, Liu S, Gao X, Nova I, Tronconi E (2020) An experimental and modelling study of the reactivity of adsorbed NH3 in the low temperature NH3-SCR reduction half-cycle over a Cu-CHA catalyst. Appl Catal B Environ 279:119397
Kwak JH, Tonkyn RG, Kim DH, Szanyi J, Peden CHF (2010) Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J Catal 275:187–190
Akter N, Han L, Huaman D, Kang Y, Kim T (2016) NO and NH3 Oxidation over zeolite materials. Mater Today Proc 3:550–555
Hünnekes EV, Van Der Heijden PVAM, Patchett JA (2006) Ammonia oxidation catalysts for mobile SCR systems. SAE Tech Pap 115:237–243
Koebel M, Elsener M, Kleemann M (2000) Urea-SCR: a promising technique to reduce NOx emissions from automotive diesel engines. Catal Today 59:335–345
Feng Y, Janssens TVW, Vennestrøm PNR, Jansson J, Skoglundh M, Gröbeck H (2021) The role of H+- And Cu+-sites for N2O formation during NH3-SCR over Cu-CHA. J Phys Chem C 125:4595–4601
Xi Y, Ottinger NA, Keturakis CJ, Liu ZG (2021) Dynamics of low temperature N2O formation under SCR reaction conditions over a Cu-SSZ-13 catalyst. Appl Catal B Environ 294:120245
Olsson L, Wijayanti K, Leistner K, Kumar A, Joshi SY, Kamasamudram K, Currier NW, Yezerets A (2015) A multi-site kinetic model for NH3-SCR over Cu/SSZ-13. Appl Catal B Environ 174–175:212–224
Hui S, Yao Q, Wang D, Niu Y (2019) Effect of oxygen on N2O and NO formation from NH3 oxidation over MnOx/TiO2 catalysts. Energy Procedia 158:1497–1501
Partridge WP, Joshi SY, Pihl JA, Currier NW (2018) New operando method for quantifying the relative half-cycle rates of the NO SCR redox cycle over Cu-exchanged zeolites. Appl Catal B Environ 236:195–204
Hu W, Iacobone U, Gramigni F, Zhang Y, Wang X, Liu S, Zheng C, Nova I, Gao X, Tronconi E (2021) Unraveling the hydrolysis of Z2Cu2+ to ZCu2+(OH)- and its consequences for the low-temperature selective catalytic reduction of NO on Cu-CHA catalysts. ACS Catal 11:11616–11625
Lomachenko KA, Borfecchia E, Negri C, Berlier G, Lamberti C, Beato P, Falsig H, Bordiga S (2016) The Cu-CHA deNOx catalyst in action: temperature-pependent NH3-assisted Selective catalytic reduction monitored by operando XAS and XES. J Am Chem Soc 138:12025–12028
Funding
No grants or other support were received for conducting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical Approval
This work does not contain any studies with human participants or animals perfomed by any of the authors.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nasello, N.D., Gramigni, F., Nova, I. et al. Transient Redox Behavior of a NH3-SCR Cu-CHA SCR Catalyst: Effect of O2 Feed Content Variation. Top Catal 66, 805–814 (2023). https://doi.org/10.1007/s11244-022-01715-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-022-01715-1