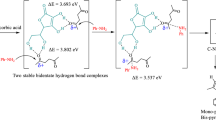
Abstract
The O–O bond activation is crucial in many oxidation reactions and dioxygen reduction reactions. Herein, we performed density functional theory (DFT) calculations to investigate how the O–O bond in a MnIV–peroxo corrole complex is activated with the addition of acid to afford the high-valent MnV–oxo corrole complex. Our results showed that protonation of MnIV–peroxo leads to the Mn–hydroperoxo complex having a hybrid MnIV–OOH and MnIII(Cor.+)–OOH character. The subsequent O–O bond cleavage is however thermodynamically unfavorable. In contrast, aided with the MnIII–corrole, the O–O bond cleavage can proceed via a binuclear mechanism to produce the MnV–oxo and MnIV–OH complexes with a small barrier and a high exothermicity. These findings provide new insights into the O–O bond cleavage for Mn–hydroperoxo complexes and could offer a clue for the development of biomimetic oxidation catalysts.









Similar content being viewed by others
References
Costas M, Mehn MP, Jensen MP, Que L (2004) Dioxygen activation at mononuclear nonheme iron active sites: enzymes, models, and intermediates. Chem Rev 104:939–986. https://doi.org/10.1021/cr020628n
Lewis EA, Tolman WB (2004) Reactivity of dioxygen-copper systems. Chem Rev 104:1047–1076. https://doi.org/10.1021/cr020633r
Solomon EI, Sarangi R, Woertink JS, Augustine AJ, Yoon J, Ghosh S (2007) O2 and N2O activation by bi-, tri-, and tetranuclear Cu clusters in biology. Acc Chem Res 40:581–591. https://doi.org/10.1021/ar600060t
Krebs C, Fujimori GD, Walsh CT, Bollinger JM (2007) Non-heme Fe(IV)–oxo intermediates. Acc Chem Res 40:484–492. https://doi.org/10.1021/ar700066p
Que L, Tolman WB (2008) Biologically inspired oxidation catalysis. Nature 455:333–340. https://doi.org/10.1038/nature07371
Lewis JC, Coelho PS, Arnold FH (2011) Enzymatic functionalization of carbon-hydrogen bonds. Chem Soc Rev 40:2003–2021. https://doi.org/10.1039/C0CS00067A
Tinberg CE, Lippard SJ (2011) Dioxygen activation in soluble methane monooxygenase. Acc Chem Res 44:280–288. https://doi.org/10.1021/ar1001473
Hohenberger J, Ray K, Meyer K (2012) The biology and chemistry of high-valent iron-oxo and iron-nitrido complexes. Nat Commun 3:720. https://doi.org/10.1038/ncomms1718
Whittaker JW (2012) Non-heme manganese catalase—the “other” catalase. Arch Biochem Biophys 525:111–120. https://doi.org/10.1016/j.abb.2011.12.008
Ray K, Pfaff FF, Wang B, Nam W (2014) Status of reactive non-heme metal-oxygen intermediates in chemical and enzymatic reactions. J Am Chem Soc 136:13942–13958. https://doi.org/10.1021/ja507807v
Solomon EI, Heppner DE, Johnston EM, Ginsbach JW, Cirera J, Qayyum M, Kieber-Emmons MT, Kjaergaard CH, Hadt RG, Tian L (2014) Copper active sites in biology. Chem Rev 114:3659–3853. https://doi.org/10.1021/cr400327t
Lee JY, Karlin KD (2015) Elaboration of copper-oxygen mediated C-H activation chemistry in consideration of future fuel and feedstock generation. Curr Opin Chem Biol 25:184–193. https://doi.org/10.1016/j.cbpa.2015.02.014
Sirajuddin S, Rosenzweig AC (2015) Enzymatic oxidation of methane. Biochemistry 54:2283–2294. https://doi.org/10.1021/acs.biochem.5b00198
Solomon EI, Park K (2016) Structure/function correlations over binuclear non-heme iron active sites. J Biol Inorg Chem 21:575–588. https://doi.org/10.1007/s00775-016-1372-9
Solomon EI, Goudarzi S, Sutherlin KD (2016) O2 activation by non-heme iron enzymes. Biochemistry 55:6363–6374. https://doi.org/10.1021/acs.biochem.6b00635
Liu JJ, Diaz DE, Quist DA, Karlin KD (2016) Copper(I)-dioxygen adducts and copper enzyme mechanisms. Isr J Chem 56:738–755. https://doi.org/10.1002/ijch.201600025
Kal S, Que L (2017) Dioxygen activation by nonheme iron enzymes with the 2-His-1-carboxylate facial triad that generate high-valent oxoiron oxidants. J Biol Inorg Chem 22:339–365. https://doi.org/10.1007/s00775-016-1431-2
Olivo G, Cussó O, Borrell M, Costas M (2017) Oxidation of alkane and alkene moieties with biologically inspired nonheme iron catalysts and hydrogen peroxide: from free radicals to stereoselective transformations. J Biol Inorg Chem 22:425–452. https://doi.org/10.1007/s00775-016-1434-z
Wise CE, Grant JL, Amaya JA, Ratigan SC, Hsieh CH, Manley OM, Makris TM (2017) Divergent mechanisms of iron-containing enzymes for hydrocarbon biosynthesis. J Biol Inorg Chem 22:221–235. https://doi.org/10.1007/s00775-016-1425-0
Faponle AS, de Visser SP (2017) The role of nonheme transition metal-oxo, -peroxo, and -superoxo intermediates in enzyme catalysis and reactions of bioinspired complexes. Adv Inorg Chem 70:167–194. https://doi.org/10.1016/bs.adioch.2017.01.002
Jasniewski AJ, Que L (2018) Dioxygen activation by nonheme diiron enzymes: diverse dioxygen adducts, high-valent intermediates, and related model complexes. Chem Rev 118:2554–2592. https://doi.org/10.1021/acs.chemrev.7b00457
Huang X, Groves JT (2018) Oxygen activation and radical transformations in heme proteins and metalloporphyrins. Chem Rev 118:2491–2553. https://doi.org/10.1021/acs.chemrev.7b00373
Banerjee R, Jones JC, Lipscomb JD (2019) Soluble methane monooxygenase. Annu Rev Biochem 88:409–431. https://doi.org/10.1146/annurev-biochem-013118-111529
Guo M, Corona T, Ray K, Nam W (2019) Heme and nonheme high-valent iron and manganese oxo cores in biological and abiological oxidation reactions. Acs Central Sci 5:13–28. https://doi.org/10.1021/acscentsci.8b00698
Battistella B, Ray K (2020) O2 and H2O2 activations at dinuclear Mn and Fe active sites. Coord Chem Rev 408:213176. https://doi.org/10.1016/j.ccr.2019.213176
Schroder D, Schwarz H, Shaik S (2000) Characterization, orbital description, and reactivity patterns of transition-metal oxo species in the gas phase. In: Meunier B (ed) metal-oxo and metal-peroxo species in catalytic oxidations, vol 97. Springer, Berlin, pp 91–123. https://doi.org/10.1007/3-540-46592-8_4
Solomon EI, Randall DW, Glaser T (2000) Electronic structures of active sites in electron transfer metalloproteins: contributions to reactivity. Coord Chem Rev 200:595–632. https://doi.org/10.1016/S0010-8545(00)00332-5
Mirica LM, Ottenwaelder X, Stack TDP (2004) Structure and spectroscopy of copper-dioxygen complexes. Chem Rev 104:1013–1046. https://doi.org/10.1021/cr020632z
Wu AJ, Penner-Hahn JE, Pecoraro VL (2004) Structural, spectroscopic, and reactivity models for the manganese catalases. Chem Rev 104:903–938. https://doi.org/10.1021/cr020627v
Nam W (2007) High-valent iron(IV)-oxo complexes of heme and non-heme ligands in oxygenation reactions. Acc Chem Res 40:522–531. https://doi.org/10.1021/ar700027f
Cho J, Sarangi R, Nam W (2012) Mononuclear metal–O2 complexes bearing macrocyclic N-tetramethylated cyclam ligands. Acc Chem Res 45:1321–1330. https://doi.org/10.1021/ar3000019
de Visser SP, Rohde JU, Lee YM, Cho J, Nam W (2013) Intrinsic properties and reactivities of mononuclear nonheme iron-oxygen complexes bearing the tetramethylcyclam ligand. Coord Chem Rev 257:381–393. https://doi.org/10.1016/j.ccr.2012.06.002
Fukuzumi S, Karlin KD (2013) Kinetics and thermodynamics of formation and electron-transfer reactions of Cu–O2 and Cu2–O2 complexes. Coord Chem Rev 257:187–195. https://doi.org/10.1016/j.ccr.2012.05.031
Nam W, Lee YM, Fukuzumi S (2014) Tuning reactivity and mechanism in oxidation reactions by mononuclear nonheme iron(IV)-oxo complexes. Acc Chem Res 47:1146–1154. https://doi.org/10.1021/ar400258p
Nam W (2015) Synthetic mononuclear nonheme iron-oxygen intermediates. Acc Chem Res 48:2415–2423. https://doi.org/10.1021/acs.accounts.5b00218
Young KJ, Brennan BJ, Tagore R, Brudvig GW (2015) Photosynthetic water oxidation: insights from manganese model chemistry. Acc Chem Res 48:567–574. https://doi.org/10.1021/ar5004175
Serrano-Plana J, Garcia-Bosch I, Company A, Costas M (2015) Structural and reactivity models for copper oxygenases: cooperative effects and novel reactivities. Acc Chem Res 48:2397–2406. https://doi.org/10.1021/acs.accounts.5b00187
Sahu S, Goldberg DP (2016) Activation of dioxygen by iron and manganese complexes: a heme and nonheme perspective. J Am Chem Soc 138:11410–11428. https://doi.org/10.1021/jacs.6b05251
Engelmann X, Monte-Perez I, Ray K (2016) Oxidation reactions with bioinspired mononuclear non-heme metal-oxo complexes. Angew Chem Int Ed Engl 55:7632–7649. https://doi.org/10.1002/anie.201600507
Quist DA, Diaz DE, Liu JJ, Karlin KD (2017) Activation of dioxygen by copper metalloproteins and insights from model complexes. J Biol Inorg Chem 22:253–288. https://doi.org/10.1007/s00775-016-1415-2
Elwell CE, Gagnon NL, Neisen BD, Dhar D, Spaeth AD, Yee GM, Tolman WB (2017) Copper-oxygen complexes revisited: structures, spectroscopy, and reactivity. Chem Rev 117:2059–2107. https://doi.org/10.1021/acs.chemrev.6b00636
Hong S, Lee YM, Ray K, Nam W (2017) Dioxygen activation chemistry by synthetic mononuclear nonheme iron, copper and chromium complexes. Coord Chem Rev 334:25–42. https://doi.org/10.1016/j.ccr.2016.07.006
Sankaralingam M, Lee YM, Nam W, Fukuzumi S (2018) Amphoteric reactivity of metal-oxygen complexes in oxidation reactions. Coord Chem Rev 365:41–59. https://doi.org/10.1016/j.ccr.2018.03.003
Adam SM, Wijeratne GB, Rogler PJ, Diaz DE, Quist DA, Liu JJ, Karlin KD (2018) Synthetic Fe/Cu complexes: toward understanding heme-copper oxidase structure and function. Chem Rev 118:10840–11022. https://doi.org/10.1021/acs.chemrev.8b00074
Guo M, Lee YM, Fukuzumi S, Nam W (2021) Biomimetic metal-oxidant adducts as active oxidants in oxidation reactions. Coord Chem Rev 435:213807. https://doi.org/10.1016/j.ccr.2021.213807
Solomon EI, Brunold TC, Davis MI, Kemsley JN, Lee S-K, Lehnert N, Neese F, Skulan AJ, Yang Y-S, Zhou J (2000) Geometric and electronic structure/function correlations in non-heme iron enzymes. Chem Rev 100:235–350. https://doi.org/10.1021/cr9900275
Rice DB, Massie AA, Jackson TA (2017) Manganese-oxygen intermediates in O-O bond activation and hydrogen-atom transfer reactions. Acc Chem Res 50:2706–2717. https://doi.org/10.1021/acs.accounts.7b00343
Baglia RA, Zaragoza JPT, Goldberg DP (2017) Biomimetic reactivity of oxygen-derived manganese and iron porphyrinoid complexes. Chem Rev 117:13320–13352. https://doi.org/10.1021/acs.chemrev.7b00180
Fukuzumi S, Lee Y-M, Nam W (2019) Structure and reactivity of the first-row d-block metal-superoxo complexes. Dalton Trans 48:9469–9489. https://doi.org/10.1039/C9DT01402K
Fukuzumi S, Cho K-B, Lee Y-M, Hong S, Nam W (2020) Mechanistic dichotomies in redox reactions of mononuclear metal-oxygen intermediates. Chem Soc Rev 49:8988–9027. https://doi.org/10.1039/D0CS01251C
Raymond J, Blankenship RE (2008) The origin of the oxygen-evolving complex. Coord Chem Rev 252:377–383. https://doi.org/10.1016/j.ccr.2007.08.026
Sheng Y, Abreu IA, Cabelli DE, Maroney MJ, Miller A-F, Teixeira M, Valentine JS (2014) Superoxide dismutases and superoxide reductases. Chem Rev 114:3854–3918. https://doi.org/10.1021/cr4005296
Goldberg DP (2007) Corrolazines: new frontiers in high-valent metalloporphyrinoid stability and reactivity. Acc Chem Res 40:626–634. https://doi.org/10.1021/ar700039y
Yin G (2013) Understanding the oxidative relationships of the metal oxo, hydroxo and hydroperoxide intermediates with manganese(IV) complexes having bridged cyclams: correlation of the physicochemical properties with reactivity. Acc Chem Res 46:483–492. https://doi.org/10.1021/ar300208z
Liu W, Groves JT (2015) Manganese catalyzed C-H halogenation. Acc Chem Res 48:1727–1735. https://doi.org/10.1021/acs.accounts.5b00062
Prokop KA, Goldberg DP (2012) Generation of an isolable, monomeric manganese(V)–oxo complex from O2 and visible light. J Am Chem Soc 134:8014–8017. https://doi.org/10.1021/ja300888t
Neu HM, Baglia RA, Goldberg DP (2015) A balancing act: stability versus reactivity of Mn(O) complexes. Acc Chem Res 48:2754–2764. https://doi.org/10.1021/acs.accounts.5b00273
Huang Z, Guan R, Shanmugam M, Bennett EL, Robertson CM, Brookfield A, McInnes EJL, Xiao J (2021) Oxidative cleavage of alkenes by O2 with a non-heme manganese catalyst. J Am Chem Soc 143:10005–10013. https://doi.org/10.1021/jacs.1c05757
Jung J, Neu HM, Leeladee P, Siegler MA, Ohkubo K, Goldberg DP, Fukuzumi S (2016) Photocatalytic oxygenation of substrates by dioxygen with protonated manganese(III) corrolazine. Inorg Chem 55:3218–3228. https://doi.org/10.1021/acs.inorgchem.5b02019
Nardis S, Mandoj F, Stefanelli M, Paolesse R (2019) Metal complexes of corrole. Coord Chem Rev 388:360–405. https://doi.org/10.1016/j.ccr.2019.02.034
Liu H-Y, Mahmood MHR, Qiu S-X, Chang CK (2013) Recent developments in manganese corrole chemistry. Coord Chem Rev 257:1306–1333. https://doi.org/10.1016/j.ccr.2012.12.017
Gross Z, Golubkov G, Simkhovich L (2000) Epoxidation catalysis by a manganese corrole and isolation of an oxomanganese(V) corrole. Angew Chem Int Ed 39:4045–4047
Golubkov G, Bendix J, Gray HB, Mahammed A, Goldberg I, DiBilio AJ, Gross Z (2001) High-valent manganese corroles and the first perhalogenated metallocorrole catalyst. Angew Chem Int Ed 40:2190–2192
Zhang R, Harischandra DN, Newcomb M (2005) Laser flash photolysis generation and kinetic studies of corrole–manganese(v)-oxo intermediates. Chem Eur J 11:5713–5720. https://doi.org/10.1002/chem.200500134
Leeladee P, Baglia RA, Prokop KA, Latifi R, de Visser SP, Goldberg DP (2012) Valence tautomerism in a high-valent manganese–oxo porphyrinoid complex induced by a lewis acid. J Am Chem Soc 134:10397–10400. https://doi.org/10.1021/ja304609n
Bougher CJ, Liu S, Hicks SD, Abu-Omar MM (2015) Valence tautomerization of high-valent manganese(V)-oxo corrole induced by protonation of the oxo ligand. J Am Chem Soc 137:14481–14487. https://doi.org/10.1021/jacs.5b09759
Neu HM, Jung J, Baglia RA, Siegler MA, Ohkubo K, Fukuzumi S, Goldberg DP (2015) Light-driven, proton-controlled, catalytic aerobic C-H oxidation mediated by a Mn(III) porphyrinoid complex. J Am Chem Soc 137:4614–4617. https://doi.org/10.1021/jacs.5b00816
Jung J, Ohkubo K, Prokop-Prigge KA, Neu HM, Goldberg DP, Fukuzumi S (2013) Photochemical oxidation of a manganese(III) complex with oxygen and toluene derivatives to form a manganese(V)-oxo complex. Inorg Chem 52:13594–13604. https://doi.org/10.1021/ic402121j
Bougher CJ, Abu-Omar MM (2016) Lewis-acid-assisted hydrogen atom transfer to manganese(V)-oxo corrole through valence tautomerization. ChemistryOpen 5:522–524. https://doi.org/10.1002/open.201600117
Kim SH, Park H, Seo MS, Kubo M, Ogura T, Klajn J, Gryko DT, Valentine JS, Nam W (2010) Reversible O-O bond cleavage and formation between Mn(IV)-peroxo and Mn(V)-oxo corroles. J Am Chem Soc 132:14030–14032. https://doi.org/10.1021/ja1066465
Guo M, Lee YM, Gupta R, Seo MS, Ohta T, Wang HH, Liu HY, Orcid DSN, Sarangi R, Fukuzumi S, Nam W (2017) Dioxygen activation and O-O bond formation reactions by manganese corroles. J Am Chem Soc 139:15858–15867. https://doi.org/10.1021/jacs.7b08678
Yu JF, Lai WZ (2021) Mechanistic insights into dioxygen activation by a manganese corrole complex: a broken-symmetry DFT study. RSC Adv 11:24852–24861. https://doi.org/10.1039/D1RA02722K
Gamba I, Codola Z, Lloret-Fillol J, Costas M (2017) Making and breaking of the O-O bond at iron complexes. Coord Chem Rev 334:2–24. https://doi.org/10.1016/j.ccr.2016.11.007
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ Gaussian 16 Revision A.03 Wallingford CT: Gaussian Inc.
Tao JM, Perdew JP, Staroverov VN, Scuseria GE (2003) Climbing the density functional ladder: nonempirical meta-generalized gradient approximation designed for molecules and solids. Phys Rev Lett 91:146401. https://doi.org/10.1103/PhysRevLett.91.146401
Jensen KP (2008) Bioinorganic chemistry modeled with the TPSSh density functional. Inorg Chem 47:10357–10365. https://doi.org/10.1021/ic800841t
Cirera J, Via-Nadal M, Ruiz E (2018) Benchmarking density functional methods for calculation of state energies of first row spin-crossover molecules. Inorg Chem 57:14097–14105. https://doi.org/10.1021/acs.inorgchem.8b01821
Ashley DC, Baik M-H (2016) The electronic structure of [Mn(V)═O]: what is the connection between oxyl radical character, physical oxidation state, and reactivity? ACS Catal 6:7202–7216. https://doi.org/10.1021/acscatal.6b01793
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305. https://doi.org/10.1039/B508541A
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001. https://doi.org/10.1021/jp9716997
Grimme S (2006) Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J Comput Chem 27:1787–1799. https://doi.org/10.1002/jcc.20495
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104. https://doi.org/10.1063/1.3382344
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comput Chem 32:1456–1465. https://doi.org/10.1002/jcc.21759
Kelly CP, Cramer CJ, Truhlar DG (2007) Single-ion solvation free energies and the normal hydrogen electrode potential in methanol, acetonitrile, and dimethyl sulfoxide. J Phys Chem B 111:408–422. https://doi.org/10.1021/jp065403l
Wang L-P, Wu Q, Voorhis VT (2010) Acid−base mechanism for ruthenium water oxidation catalysts. Inorg Chem 49:4543–4553. https://doi.org/10.1021/ic100075k
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38:3098–3100. https://doi.org/10.1103/PhysRevA.38.3098
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Becke AD (1993) Density-fuctional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: the PBE0 model. J Chem Phys 110:6158–6170. https://doi.org/10.1063/1.478522
Ernzerhof M, Scuseria GE (1999) Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J Chem Phys 110:5029–5036. https://doi.org/10.1063/1.478401
de Visser SP, Ogliaro F, Gross Z, Shaik S (2001) What is the difference between the manganese porphyrin and corrole analogues of cytochrome P450’s compound I? Chem Eur J 7:4954–4960
Ganguly S, McCormick LJ, Conradie J, Gagnon KJ, Sarangi R, Ghosh A (2018) Electronic structure of manganese corroles revisited: X-ray structures, optical and X-ray absorption spectroscopies, and electrochemistry as probes of ligand noninnocence. Inorg Chem 57:9656–9669. https://doi.org/10.1021/acs.inorgchem.8b00537
Zhao H, Pierloot K, Langner EHG, Swarts JC, Conradie J, Ghosh A (2012) Low-energy states of manganese-oxo corrole and corrolazine: multiconfiguration reference ab initio calculations. Inorg Chem 51:4002–4006. https://doi.org/10.1021/ic201972f
Alcover-Fortuny G, Caballol R, Pierloot K, de Graaf C (2016) Role of the imide axial ligand in the spin and oxidation state of manganese corrole and corrolazine complexes. Inorg Chem 55:5274–5280. https://doi.org/10.1021/acs.inorgchem.6b00194
Shaik S, Cohen S, Wang Y, Chen H, Kumar D, Thiel W (2010) P450 enzymes: their structure, reactivity, and selectivity-modeled by QM/MM calculations. Chem Rev 110:949–1017. https://doi.org/10.1021/cr900121s
Cho KB, Hirao H, Chen H, Carvajal MA, Cohen S, Derat E, Thiel W, Shaik S (2008) Compound I in heme thiolate enzymes: a comparative QM/MM study. J Phys Chem A 112:13128–13138. https://doi.org/10.1021/jp806770y
Acknowledgements
The work is supported by the grants from National Natural Science Foundation of China (No. 21673286). The computer resources were provided by High-performance Computing Platform of Renmin University of China.
Supporting Information
Calculated electronic energies, zero-point energy corrections, thermal corrections, spin densities, and Cartesian coordinates of all computed species
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Ethical Approval
This study does not require an ethics committee.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, J., Wang, Y., Yang, Y. et al. Mechanistic Insight into the O–O Bond Activation by Manganese Corrole Complexes. Top Catal 65, 493–504 (2022). https://doi.org/10.1007/s11244-021-01525-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11244-021-01525-x