Abstract
The partial differential equations describing immiscible, but soluble, carbon dioxide (CO2) displacement of brine in saline storage formations are developed including mass transfer across the CO2–brine interface. Scaling relationships for characteristic time among laboratory and representative storage formation conditions are found upon assumption that free-phase CO2 transport during injection is dominated by pressure-driven flow. The implication is that an hour in the FluidFlower (room-scale visual model) scales to hundreds of years of elapsed time in the storage formation. The scaling criteria permit extrapolation of the effects of changes in parameters and operating conditions. Interphase mass transfer allows CO2 to saturate the brine phase and the finite time of such mass transfer results in substantial time to approach equilibrium. Significant mixing of CO2 dissolved into formation brine with original brine is found experimentally and is also predicted. The magnitude of onset time for buoyancy-driven fingers that enhance mixing of CO2 is typically only a fraction of the duration of CO2 injection and in general agreement with theoretical analysis in the literature. Predictions for onset time of convective mixing at representative storage formation conditions, likewise, teach that the onset time for fingering is significantly less than the duration of CO2 injection in some cases. The implications of this observation include that mixing of CO2 with brine and the subsequent settling due to gravity are relatively rapid and coincide with the period of active CO2 injection.
Similar content being viewed by others
1 Introduction
Predictions of the future energy system include significant geological carbon storage operations where carbon dioxide (CO2) is injected into deep, porous, saline aquifers to decrease atmospheric emissions (IEA 2020). Moreover, capture of CO2 and geological storage may be the most rapid and cost-effective means to address emissions from hard to abate industries such as cement manufacture. Accurate forecasting of the short- and long-term performance of geological carbon storage operations in complex geological media requires good understanding of the underlying coupled processes.
The “FluidFlower” is an important new experimental tool for exploring coupled transport, physical, and nonequilibrium processes accompanying carbon dioxide (CO2) injection into saline storage formations with complex geological bedding including faults and folds (Fernø et al. 2023). It is a room-scale (2.8 m long by 1.3 m tall), two-dimensional, physical model filled with sand and is the subject of a special issue in this journal. See (Eikehaug et al. 2023, 2024; Haugen et al. 2024) for extensive details of the experimental setup and repeatability of results, respectively. One of the primary outcomes of experiments conducted in the FluidFlower is the spatial evolution of injected CO2 as a free phase as well as dissolved in brine (Flemisch et al. 2023). FluidFlower results have already been used to benchmark the performance of various simulators for geological carbon storage against experimental data (Flemisch et al. 2023; Jammoul et al. 2023; Wang et al. 2023) and assess uncertainties in numerical modeling (Green et al. 2023; Wapperom et al. 2023) and as the basis for a digital twin of carbon storage operations (Keilegavlen et al. 2023). The FluidFlower has also been used to understand the value of particular prior knowledge on the forecasting ability of history-matched simulations (Saló-Salgado et al. 2023). In addition to the above work reported in the special issue, FluidFlower results may also be useful for comparison to analytical and numerical solutions for sloping, confined and unconfined storage formations (Gupta & Bryant 2010; Hesse et al. 2006, 2008; Juanes et al. 2010; Juanes and MacMinn 2008; Vella and Huppert 2006; Callas et al. 2023). Many of these papers do not include secondary/residual trapping and other physics that may, or may not, speed up immobilization of CO2 (Golding et al. 2017).
Viewing the interplay of CO2 transport with geological heterogeneity allows researchers to communicate to a wide community the mechanisms by which CO2 may be stored long term as well as the types of geological features that promote secure storage. In this sense, the FluidFlower follows a long tradition among the flow in porous media community of scaled physical models to understand complex coupled processes—for example, Werner et al. (2014) and Trevisan et al. (2017) for geological carbon storage and Basu and Islam (2009) for chemical injection processes. Important aspects of such experiments include translation of experimental time into an equivalent time in the subsurface, and importantly, an understanding of how processes such as the rate of interphase mass transfer and convective mixing of CO2-laden brine differs between the physical, laboratory model and the field.
The subsurface engineering community is rich with studies where scaling criteria have been developed to understand laboratory results and high-fidelity numerical model results in the context of field applications (Table 1). Mass transfer across the CO2–brine interface during geological storage, for instance, is of importance because the aqueous phase is not initially saturated with CO2 (Erfani et al. 2022; Lindeberg & Wessel-Berg 1997; Weir et al. 1995) and vertical flow equilibrium (Yortsos 1995) may not result during injection. The rate of dissolution of CO2 into brine is controlled by diffusion, but, importantly, the resulting denser fluid may settle downward in the storage formation under the action of gravity, so-called convective mixing, thereby enhancing solubility trapping of CO2 (Ennis-King and Paterson 2005; Hassanzadeh et al. 2007; Kneafsey and Pruess 2010; Riaz et al. 2006) where the onset of convective mixing may depend on dispersion (Hidalgo and Carrera 2009). Additionally, capillarity and the capillary transition zone appear to be important to the timing of the beginning of convective mixing and lead to a range of outcomes (Elenius et al. 2012; MacMinn et al. 2011).
There are also substantial scaling studies in the oil recovery domain with learnings that cross into carbon storage. For example, Lozada and Farouq Ali (1987) examine the displacement of heavy oil by immiscible carbon dioxide and the mass transfer of CO2 into liquids to understand the role of different operating conditions. Additionally, Basu and Islam (2009) and Islam and Farouq Ali (1990) present studies of scaling among laboratory and field for chemical enhanced oil recovery. In many cases, porous media properties and pressure differ significantly from the field (Kimber and Ali 1989). Table 1 presents a breadth of physical and chemical mechanisms addressed by previous studies across domains.
The general consensus is that it is practically very difficult to scale all operative mechanisms in experiments with complex physical and chemical processes, but it is possible to estimate time scaling with a degree of certainty in systems with pressure- and/or buoyancy-driven flow as well as to understand the differences in scaling between laboratory and field for interphase mass transfer, diffusive transport within a phase, gravity, and so on.
With this backdrop, this manuscript is the first to present a methodology for and quantification of the scaling of time and physical processes in the FluidFlower and a geological formation during CO2 injection. Local thermodynamic equilibrium is not assumed, nor is CO2 dissolution during the injection period neglected, because results from the FluidFlower have exhibited mass transfer effects at the gas/brine interface as well as convective mixing of CO2-laden brine. The important questions that we answer are (i) is transport of CO2 in response to pressure gradients relevant at both laboratory and field scale, (ii) how is time scaled between these two settings, and (iii) can convective mixing of CO2-saturated brine begin during injection?
We proceed with a description of the FluidFlower apparatus, the specific storage zone that is analyzed, the mathematical model and simplifications, model nondimensionalization, and the scaling groups that emerge. The scaling groups are then computed for laboratory and representative field conditions to assess that the main transport mechanism in laboratory results and storage formations is pressure or buoyancy-driven flow. Time scales, the relative importance of gravity between the laboratory and the field, rates of dissolution of CO2 into brine, the wavelength of unstable CO2-saturated brine fingers that move downward by gravity, and so on are used to explore similarities and differences among physical processes in the FluidFlower and the field. Discussion and conclusions complete the paper.
2 FluidFlower Overview
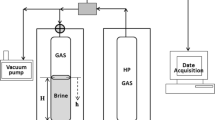
The FluidFlower is a room-scale (2.8 m long by 1.3 m tall) physical model capable of representing features found in a vertical geological cross section. Development and geometry of the apparatus are detailed in Sects. 2 and 3 of Eikehaug et al. (2023, 2024) and Sect. 2 of Fernø et al. (2023). The front is transparent Plexiglas permitting visual observation, whereas the back is opaque with 56 ports for fluid injection and pressure monitoring. The side and bottom boundaries are closed, while the top boundary is open to the atmosphere. The FluidFlower is packed with unconsolidated sands of varying grain size, as described in Sect. 2.3 of Fernø et al. (2023), to create different geometries of porous media structures resembling geological strata including trapping structures, faults, and folds. Haugen et al. (2024) analyze and discuss the variability in the resulting packing. Once sands are installed, the model is flushed numerous times to settle sand grains and improve repeatability, Sect. 2.3 of Fernø et al. (2023). Experiments are conducted at room temperature.
Section 1 of Haugen et al. (2024) describes measurement of porosity, permeability, end point relative permeability and capillary entry pressures for each sand type with properties summarized in their Table 1. The nominal porosity is 0.44 for all sands, and permeability ranges from 50 to 4000 Darcy for the various sands (Haugen et al. 2024). The physical model is quasi two-dimensional, with nominal thickness from 19 mm (closed boundaries) up to more than 25 mm in the central regions (depth map provided in Fernø et al. 2023). The thickness of the model is at least 8–10 times greater than the maximum grain size to mitigate poor flow conditions (Eikehaug et al. 2023, 2024). Uniform porosity is assumed in each sand layer and across the width, and local widths are used to calculate the pore volume in each sand layer.
Figure 1 shows a 2.3 m long by 0.6 m tall subregion that is a storage formation and the focus of this manuscript. Refer to Fig. 1 of Fernø et al. (2023) for an overview of the entire geometry of the model. The lightly colored sealing layer in Fig. 1 is composed of fine-grained sand, overlaying the coarser storage reservoir sand. The seal prevents gas entry up to about a gas column height of 0.2 m at pressure conditions near atmospheric whereas the maximum height of the gas zone is 0.1 m given the anticlinal geometry of the barrier layer and the open boundaries. This maximum height is illustrated in Fig. 1 (c) where free-phase CO2 spills upward around the left edge of the storage zone. This particular configuration of sands was used for five repeat experiments and showed excellent reproducibility as detailed in Sects. 3.2.1 to 3.2.3 of Fernø et al. (2023) and 2.6 of Haugen et al (2024).
Representative images of filling of storage zone, saturation of underlying brine with dissolved CO2, and fingering of CO2-laden brine into CO2-free brine in the FluidFlower. The injection point is marked with a blue circle. In image a taken at 34 min post the start of CO2 injection, diffusion/dispersion of CO2 into the brine below the gas cap is evident with possible indications of initiation of fingers as shown by inset image, b taken at 105 min displays expansion of the gas-filled zone and fingers, while c taken at 647 min shows well-developed miscible fingers and CO2 spilling at the left boundary. The constant number of fingers in (b) and (c) suggests that little coalescence of fingers occurs over these time scales
The injection point for gaseous CO2 is marked with a blue circle on the left of Fig. 1. Injection is via a mass flow controller, as described in Sect. 4 of Eikehaug et al. (2023, 2024), at a rate of 10 cm3/min at standard conditions (Fernø et al. 2023). The free-phase CO2 is injected in a high permeability reservoir sand (4000 Darcy); injection pressure is about 1000 Pa above atmospheric and atmospheric pressure varies among repeated experiments. The aqueous phase is distilled water with 0.67 mN NaCl and equivalent concentrations of three pH-sensitive dyes. The compositions of the various fluids are presented in Table 1 of Fernø et al. (2023), and the selection process for aqueous-phase dyes is discussed in Sect. 5 of Eikehaug et al (2023, 2024). Henceforth, we refer to the aqueous phase in the FluidFlower as brine. Buoyant free-phase CO2 (appears orange-red color) accumulates beneath the seal and displaces the aqueous phase.
It takes 250 min to fill the trap in Fig. 1 until CO2 spills out of the left side, the total injection time is 5 h (300 min), and the total time of observation is 120 h (5 days). The average rate of advance of the CO2-phase/aqueous phase interface prior to spill is 7 × 10–6 m/s (0.6 m/d). Free-phase CO2 (in the region studied under the anticline) does not move due to injection or buoyancy after injection stops. Another notable aspect of Fig. 1 is the dissolution of CO2 into the brine underlying the region containing free-phase CO2. This CO2 laden brine takes on a deep red color that is nearly carmine. At late times as shown in Fig. 1c, this dense CO2-laden brine falls downward through less dense CO2-free brine exhibiting miscible fingers. The technique of pH sensitive dyes has not been refined sufficiently to date to yield mass fractions in the aqueous phase. Current work is ongoing to identify better choices of pH sensitive dye.
A high-resolution camera (7952 × 4472 pixels) acquires time-lapse images at 10 s to 5 min intervals depending upon how rapidly the distribution of phases is evolving, see Sect. 2.6 of Fernø et al. (2023). An experiment results in roughly 1000 images and the results of a typical experiment are available elsewhere for download (Eikehaug et al. 2023). An open-source image analysis toolbox DarSIA, short for Darcy Scale Image Analysis (Both et al. 2023a, 2023b; Nordbotten et al. 2023), provides the capability to extract physically interpretable data from the time-lapsed images of CO2 injection and storage experiments. A set of assumptions enabled the phase quantification:
-
I.
gas-filled regions are 100% saturated with the gas (CO2),
-
II.
CO2 concentration in the CO2-saturated brine is constant, and
-
III.
the interface between the gas accumulation and liquid is sharp.
The reader is referred to Sect. 2.7 of Fernø et al. (2023) for a detailed discussion of all image-analysis assumptions.
Based on these assumptions, a two-staged geometric separation of the formation water from any CO2 in the system and of the gaseous CO2 from the CO2-saturated water is sufficient. Through pixel-wise image comparison to the image corresponding to the injection start, a thresholding approach both in the CMYK and RGB color spaces enables phase separation. A constant phase saturation in the third dimension is assumed. Facies-based threshold parameters are carefully tuned through visual identification of the respective distinct plumes and their boundaries, based on several calibration images from all experimental runs.
The FluidFlower is primarily a two-dimensional device because the depth is much less than the height and the width of the model. Hence, it is interesting to compare differences between simulated two-dimensional and three-dimensional behavior of fingers. Prior studies have revealed only modest differences in the time needed to form fingers as well as the downward flux of CO2 mass (Pau et al. 2010). The third dimension did increase the complexity of fingers formed; however, the cumulative CO2 mass flux was only about 25% greater for three-dimensional as compared to two-dimensional cases. This difference was viewed as small in comparison to the unknown and typically large variation in permeability subsurface that raises greater uncertainty in results. Hence, two-dimensional geometries are useful to understand storage formation dynamics. On the other hand, the storage formation geometry in Fig. 1 accentuates the vertical dimension somewhat. The ratio of the height to the width in the storage zone is about 0.05 in the FluidFlower, whereas characteristic height and width from storage formations in Table 2 and references therein (Northern Lights and Sleipner) yield ratios of about 0.02. Hence, the vertical dimension is exaggerated by a factor of about 2–3.
3 Model Description
This section presents a first-order model for processes in the FluidFlower. The analysis is limited to the storage zone shown in Fig. 1 and is two-dimensional. We progress from the main simplifications introduced to the dimensionless model to the scaling groups that emerge. It is assumed that the reader is acquainted with dimensional analysis and ordering, e.g., (Barenblatt 1996; Denn 1980).
3.1 Main Simplifications
The following simplifications were made in the development of dimensionless equations and groups.
1. The variation of temperature is small across the system and so conditions are taken as isothermal.
2. There are two components denoted as w and c for water and carbon dioxide, respectively.
3. There are two phases in which the two components are mutually soluble. These phases are b and g denoting the brine-rich and CO2-rich phases, respectively.
4. The multiphase extension of Darcy’s law describes the flow, u, of a phase, β, as
where k is the permeability, krβ is the relative permeability, μβ is the viscosity, pβ is the pressure, \({\varrho }_{\beta }\) is the phase mass density, and g is the acceleration of gravity.
5. The dispersive flux of a component, Ji, in partially saturated porous media is described as (Ogata & Banks 1961)
where ϕ is porosity, Sβ is the phase saturation, ρβ is the phase molar density, Diβ is the dispersion coefficient tensor, and χiβ is the mole fraction of component i in phase β. Clearly, Sβ and χiβ each sum to 1.
6. Mass transfer between phases is described by a two-film interface model as shown in Appendix A.
7. There is no sorption of components to solids.
8. There are no chemical reactions.
9. The analysis is applicable to a two-dimensional vertical cross section.
In view of the near atmospheric pressure in the FluidFlower and small gas-phase viscosity, assumption of an inviscid CO2 phase and then proceeding to a material balance is appealing as an additional simplification. Accordingly, the magnitude of kinematic viscosity (ν = μ/\(\varrho\)) was evaluated for each phase because mass flux (ρβuβ) of a phase is inversely proportional to ν. At FluidFlower conditions, νg is 8 × 10–6 m2/s whereas νb is 1 × 10–6 m2/s. At conditions approximating a storage formation (2.6 × 107 Pa and 366 K), νg and νb are 8 × 10–8 and 3 × 10–7 m2/s, respectively. The similar magnitudes of ν, the relatively modest differences in ν between phases, and the crossover in the phase with maximum ν as pressure and temperature increase suggest that, while the CO2 phase is quite mobile, CO2 kinematic viscosity is not negligible in comparison to brine.
Additionally, the assumption of an inviscid gas phase in porous media is sometimes made when the gas in pores is in communication with a large volume of gas (i.e., the atmosphere). Thus, pressure does not build up in the gas phase or pressure equilibrates nearly instantaneously. Here, this is not the case. Gas is injected and does not form a phase connected to a constant pressure source. For this reason, gas flow must be taken into account.
3.2 Phase Flux
Equation (1) is rewritten before proceeding to a mole balance on CO2 and brine components. Below, flow is described separately according to pressure gradient and gravity forces. To proceed, we express the hydrostatic pressure, \({P}_{\beta }\), as
where the integration is from the bottom "surface" of the storage zone. That is, the base of the storage zone that is the position of brine–gas interface (Fig. 1c). The symbol \({\overline{\varrho } }_{\beta }\) is the mass density of the fluid phase at depth z taken at hydrostatic pressure for brine and static conditions for the gas phase. Then, subtracting and adding Pβ to Darcy's law, Eq. (1), as well as evaluating the gradient of terms with gravity yields
Equation (4) expresses the phase flux as with reference to the deviation from hydrostatic conditions. Both pressure difference and density difference terms tend to zero as the system approaches static equilibrium.
3.3 Dimensionless Mole Balance
The nondimensionalized mass balance of component "i" incorporating multiphase transport of multicomponent fluids by bulk-phase flow and dispersion in the FluidFlower is obtained by summing over phases b and g and considering transport in the x- and z-direction. The relevant time scale for nondimensionalization is the time for vertical flow due to the imposed pressure gradient. The reference length scale is the vertical height of the domain, and the reference permeability is the vertical component. The result is
where the z-direction is aligned with the direction of gravity. The subscript D denotes a quantity that has been nondimensionalized, the subscript R marks characteristic quantities, L is the characteristic horizontal length, H is the characteristic vertical dimension, Diβ is the dispersion coefficient of component i in phase β, DR is a representative magnitude of dispersion, gR is the magnitude of gravitational acceleration, gD indicates the direction of gravity, and qiD is the nondimensionalized source/sink term for component i. The ratio \(\frac{{\left({\it{k}}{k}_{rg}\right)}_{RH}}{{\left({\it{k}}{k}_{rg}\right)}_{RV}}\) expresses the anisotropy in the effective permeability of phase "g" as the effective permeability in the horizontal dimension upon the effective vertical permeability. The spatial variable z is nondimensionalized by H, whereas xD is equal to x/L.
Flow due to pressure gradient and gravity are separated in Eq. (5) to make subsequent evaluation of the magnitude of these driving forces relative to each other more straightforward. Nondimensionalization of gradient terms is achieved via differences in pressure and density. The dimensionless phase potential is taken as \({\Pi }_{\beta D}=\left({p}_{\beta }-{P}_{\beta }\right)/\left({p}_{gR}-{p}_{g0}\right)\) where \({p}_{gR}\) is the average stabilized pressure in the formation resulting from injection that depends on rate and \({p}_{g0}\) is the average initial pressure. The reference density difference is evaluated as \({\Delta \varrho }_{gR}={\varrho }_{g}\left({p}_{gR}\right)-{\varrho }_{g}\left({p}_{g0}\right)\) consistent with ПβD and \({\Delta p}_{gR}={p}_{gR}-{p}_{g0}\). Hence, \({\varrho }_{\beta D}-{\overline{\varrho } }_{\beta D}(z)\) is equal to \(({\varrho }_{\beta }-{\overline{\varrho } }_{\beta }(z))/{\Delta \varrho }_{gR}\).
The dimensionless groups of \(\frac{{\Delta \varrho }_{gR}{g}_{R}H}{{\Delta p}_{gR}}\), \(\frac{{t}_{R}{D}_{R}}{{L}^{2}}\), and so on help us to understand the relative importance of flow driven by gravity and dispersive transport respectively. Interphase mass transfer does not appear in Eq. (5) because mass of species "i" lost by one phase is balanced exactly by the mass gained by the other phase and so it is considered separately in a later section.
The characteristic time, tR, was obtained during nondimensionalization by making the coefficient on the expression for flow due to the pressure gradient in the vertical direction, that is the second term on the right of Eq. (5), to be of order 1. Hence, the characteristic time is
where (kkrg)R is a characteristic permeability to the gas. This choice of characteristic time makes dimensionless mass accumulation and z-direction flow to be of order 1 and, consequently, asserts that bulk-phase flow driven by the pressure gradient is the main transport mechanism during injection. This assertion is tested later by considering the magnitude of the coefficients of Eq. (5) using representative physical quantities. Note also that the inverse of the ratio \({\mu }_{gR}H/{\left(k{k}_{rg}\right)}_{RV}{\Delta p}_{gR}\) defines a characteristic vertical Darcy velocity. The characteristic source/sink term follows as
Equation (5) gives a fundamental constraint on the dynamics of both the FluidFlower as well as field-scale systems. On the other hand, it is important to note that Eq. (5) by itself does not provide a closed system, but must be complemented by a phase partitioning model, constitutive relations, boundary conditions, and so on. The phase partitioning will, in itself, introduce a characteristic time scale, as is discussed below in the context of interphase mass transfer.
4 Scaled Processes
In practice, it is very difficult to scale all processes between the laboratory and the field when (i) the coupled physical processes are complex and (ii) the geometry and permeability of the porous medium are heterogeneous (Lozada and Ali 1987). The aim of this section is to establish the scaling of time between the FluidFlower and the storage formation for flows driven by pressure gradient and gravity. Importantly, we estimate the differences in the relative magnitudes of transport driven by gravity and dispersion as well as mass transfer from the CO2-rich phase to the brine-rich phase. This analysis applies to conditions during injection and before CO2 spills (Fig. 1).
4.1 Time Scaling Between Lab and Field
The relation between elapsed time in the storage formation, tForm, and that in the FluidFlower, tFlow, is determined by the ratio of characteristic times as
The characteristic time for the FluidFlower is obtained from Eq. (6) and used to scale experimental results between cases with parameters approximating the Northern Lights (Marashi 2021), Sleipner (Chadwick 2013; Chadwick et al. 2012), and In Salah projects (Bissell et al. 2011; Ringrose et al. 2009).
Table 2 lists the data used to describe these field projects and the FluidFlower case shown in Fig. 1. Settling of sand is reported as described above. The porosity of the storage formation in the FluidFlower was reduced for observed sand settling from 0.44 to 0.40 consistent with the fractional reduction in sand column height. The permeability was then estimated using the Carmen–Kozeny equation (Lake et al. 2014). The sand permeability of 4.26 × 10–9 m2 from (Haugen et al. 2024) was, thus, reduced to 2.79 × 10–9 m2. Additionally, storage formation heights were multiplied by the net-to-gross ratio (ratio of sand thickness to the overall height of the formation).
With the values in Table 2, Eq. (8) teaches that 1 h in the FluidFlower is representative of hundreds of years in the formation as summarized in Table 3. For Northern Lights and Utsira conditions, an hour in the FluidFlower scales to about 400 years whereas for In Salah conditions an hour scales to about a hundred years. The differences are primarily affected by formation thickness and permeability.
The time to fill the storage layer in the FluidFlower such that CO2 spills out of the trap is roughly 4 h (250 min) at the given injection rate (Fig. 1). With the values in Table 2 and Eq. (6), the experimental time to spill scaled to storage formation conditions is 100's to 1000's of years. Simulations of storage at Northern Lights (Johansen formation) injected CO2 at a rate of 1.6 Mt/y and the storage formation, as simulated, had a capacity of 21,680 Mt (Marashi 2021). The time to fill the storage formation at this rate is about 6000 years. The long times required to fill a large-capacity storage formation are consistent with tForm/tFlow in Table 3.
4.2 Horizontal Flow
The magnitude of the coefficient \({\left(\frac{H}{L}\right)}^{2}\left(\frac{{\left({\text{k}}{k}_{rg}\right)}_{RH}}{{\left({\text{k}}{k}_{rg}\right)}_{RV}}\right)\) on the first term on the right of Eq. (5) captures the relative importance of pressure gradient-driven flow in the horizontal direction. With values in Table 2 for the pressure increase due to injection, ∆pgR, of the FluidFlower and the Northern Lights project, this coefficient is 0.006 and 0.0003, respectively, indicating that the vertical direction dominates during pressure-driven transport. Results for other cases are found in Table 3. Note the importance of the characteristic horizontal dimension to results. Decreasing L from 10,000 to 1000 m for the Utsira case increases the coefficient from 0.002 to 0.2. The summary in Table 3 supports the importance of flow in the vertical direction.
4.3 Gravity-Driven Flow
The importance of gravity as a force for driving bulk-phase flow in the FluidFlower and the field is understood by computing the coefficient \(\frac{{\Delta \varrho }_{gR}{g}_{R}H}{{\Delta p}_{gR}}\) in front of the third term on the right of Eq. (5). The magnitude of the coefficient as compared to a value of 1 instructs about the relative importance of gravity. Likewise, the ratio of the coefficient is a measure of the difference in the importance of gravity between the storage formation and the FluidFlower. Values from Table 2 are used again, and results for the 4 cases are in Table 3. The density differences in Table 2 are computed from the density evaluated at the stabilized injection pressure and the average initial formation pressure.
The magnitude of \(\frac{{\Delta \varrho }_{gR}{g}_{R}H}{{\Delta p}_{gR}}\) for the FluidFlower is 2 × 10–5. This value is indicative of the importance of flow driven by pressure gradient in the FluidFlower. The coefficient rises to values of 0.008 and 0.06 for conditions representative of Northern Lights and Utsira storage formations, respectively, indicating that the role of gravity relative to pressure is greater in the field as compared to the FluidFlower. Likewise, that ratio of the coefficients for gravity (FluidFlower: storage formation) is of order 0.001 indicating that the FluidFlower under represents the role of gravity with respect to the storage formation during injection. That is, the influence of gravity segregation on mass transport is greater in the field. Importantly, the values of \(\frac{{\Delta \varrho }_{gR}{g}_{R}H}{{\Delta p}_{gR}}\) less than 1 are indicative that pressure gradient contributes significantly to transport during active injection.
4.4 Dispersive Transport
The magnitude of the coefficient \(\frac{{t}_{R}{D}_{gR}}{{H}^{2}}\) preceding the fifth term on the right of Eq. (5) teaches about the relative importance of dispersive transport of a component in the z-direction. This section specifically examines dispersive transport of CO2 in the brine phase as it is relevant to understand the miscible fingers in Fig. 1c. The characteristic times tRForm and tRFlow developed earlier for the FluidFlower and Northern Lights examples are used. The diffusivity of CO2 in the brine phase is taken as 1.9 × 10–9 m2/s at FluidFlower conditions (Tamimi et al. 1994) and 7.0 × 10–9 m2/s at Northern Lights conditions (Cadogan et al. 2014).
The dispersion coefficient is obtained from a compilation of measurements by Jha et al. (2011), Lake et al. (2014). Specifically, we use Fig. 11 of Jha et al. that plots the ratio of dispersion coefficient upon diffusion coefficient (\({D}_{c}/{\mathcal{D}}_{c}\)) versus velocity that is made dimensionless by the ratio of particle diameter upon diffusion coefficient. The average interstitial velocity in the z-direction of the gas/liquid interface in the FluidFlower is obtained from images in Fig. 1 and dividing by the ϕR and SgR in Table 2 as 1.9 × 10–5 m/s (= 7 × 10–6/(0.4)(0.88)). The particle diameter of the storage zone in Fig. 1 is 1.77 mm such that \({D}_{c}/{\mathcal{D}}_{c}\) for CO2 in the brine phase is found as 10 and the dispersion coefficient is 1.9 × 10–8 m2/s. Similarly, taking the interstitial velocity at Northern Lights conditions as 0.1 m/d (6.6 × 10–6 m/s), the particle diameter as 32 µm, and the diffusivity above yields \({D}_{c}/{\mathcal{D}}_{c}\) of about 1. We assume that this ratio is roughly 1 for the other cases as well. We caution that these dispersion coefficients are rough estimates and with some heterogeneity they may differ by an order of magnitude.
With these values, we find that \(\frac{{t}_{R}{D}_{gR}}{{H}^{2}}\) is equal to 8 × 10–7 in the FluidFlower and ranges from 10–7 to 10–6 in the various storage formations. The small values are consistent with macroscopic transport being driven primarily by bulk-phase flow. Values of \(\frac{{t}_{R}{D}_{gR}}{{L}^{2}}\) are even smaller because L is at least on order of magnitude greater in all cases.
4.5 Interphase Mass Transfer
Diffusion, while not a contributor to transport over large distances, is quite important to driving mass transfer across the interface between phases. Note the carmine-red layer of CO2-laden brine beneath the gas zone in Fig. 1b. Subsequently, locally dense brine phase sinks vertically through the model. Interphase mass transfer of CO2 does not appear in the overall mole balance for chemical species, Eq. (5), because mass lost by the CO2-rich phase is equal to the mass gained by the brine phase.
To understand mass transfer rates within the zone occupied by CO2 and brine, we apply the so-called two-film model for mass transfer resistance at the interface between phases to quantify the mass transfer rate (Lewis & Whitman 1924). Appendix A shows that the flux of CO2 across the interface between the CO2-rich phase and brine phase, Jcgb, is written as
where K is an overall mass transfer coefficient, ai is the interfacial area, χ is again mole fraction, the subscript ci refers to the amount of CO2 in the brine phase at interface conditions, and the subscript cb refers to CO2 in the bulk brine phase.
Martin et al. (1981) measured the mass transfer between CO2 and liquid phases in porous media under immiscible conditions and present a correlation for mass transfer resistance that is a function of CO2 flow rate. We use this correlation and modify it as suggested by Lozada and Ali (1987) and include the pressure drop. That is, we apply Darcy's law to describe mass flux as well as divide by porosity and liquid phase saturation to obtain the interstitial phase velocity, vw. The expression for mass transfer coefficient is
or
where B is determined by experiment (Martin et al. report 0.011). The diffusivity of CO2 in brine, \({\mathcal{D}}_{cb}\), is the molecular diffusivity of CO2 in the liquid phase accounting for the pore-scale nature of mass transfer from g to b phases.
The way ahead is to compute the ratio of interphase mass transfer as given by Eq. (9) between the FluidFlower and the representative formations. We set χcb equal to 0 in Eq. (9) because the largest mass transfer rates are experienced where the amount of CO2 dissolved in the brine is small. The equilibrium solubility of CO2 in brine at the interface (\({\chi }_{ci}\)) is computed as described by Enick and Klara (1990) using their correlations and the Krichevsky–Ilinskaya equation (Prausnitz et al. 1999). The solubility is found to be \({\chi }_{ci}=0.021\) at Northern Lights conditions and \({\chi }_{ci}=6.8 x{ 10}^{-4}\) at FluidFlower conditions. The prediction for FluidFlower conditions agrees well with data (\({\chi }_{ci}=\) 7 × 10–4) in Lange's Handbook (Lange 2017).
Equation (10) is substituted into Eq. (9) and the ratio of mass transfer in the FluidFlower relative to the storage formation is found. In this way, the coefficient B does not need to be evaluated. Parameters for calculation are taken from Table 2 and supplemented by Table 4. Equation (10a) is used for the FluidFlower, whereas Eq. (10b) is used for the storage formation. It is anticipated that nonzero mass transfer from the gas to the brine phase occurs at the advancing interface in the storage formation because elapsed time is much greater and, hence, Eq. (10b) is more applicable. The interstitial brine velocity for the storage formation conditions is set to 6.6 × 10–6 m/s consistent with the earlier discussion of dispersion.
The ratio between FluidFlower and storage formation conditions is roughly a factor of 30 for Northern Lights, 300 for Sleipner conditions, and about 3 for In Salah. These ratios greater than 1 primarily result because the permeability of the sands in the FluidFlower are 4 orders of magnitude larger than the storage formation and the values of H differ by at least 2 orders of magnitude. The calculations summarized in Table 4 indicate that interphase mass transfer is faster in the FluidFlower relative to the formation and motivate the exploration of fingering and convective mixing that follows.
5 Fingering and Convective Mixing
An outcome of the mass transfer described by Eq. (9) is the formation of a layer of dense CO2-laden brine just beneath the gas-brine interface in the FluidFlower in Fig. 1. This CO2-laden brine clearly segregates downward convectively in Fig. 1c. Mixing of CO2 and brine involves mass transfer of CO2 to the gas-brine interface, diffusion of CO2 away from the interface and into the bulk brine, and the convection of denser CO2-laden brine downward in the formation through the formation of fingers. The downward motion of dense brine and removal of CO2 from the capillary transition region increases the rate of dissolution, where the capillary transition is the region in which saturations transition from a mixture of CO2 and brine to only brine. This interface is dispersed due to the interfacial tension between CO2 and brine.
Figure 2 presents an overview of dissolution of CO2 versus time into the brine phase as found in the FluidFlower using time-lapse images. First, the volume of free-phase CO2 is found using the image analysis toolbox DarSIA (Nordbotten et al. 2023) with the assumptions outlined above and procedure detailed elsewhere (Both et al. 2023b). Recall that local thickness variations in the storage formation are accounted for in the mass calculations. Then, the dissolved mass is obtained by subtracting the mass of CO2 in the free phase from the cumulative injected mass. The mole fraction of the CO2 phase is assumed to be 1. Figure 2 also plots the diffusive limit as a dashed line computed using \({\mathcal{D}}_{cb}\) from Table 2. Note the inset that presents results at very short times. The thickness of a CO2 saturated layer is of order \(2(\mathcal{D}t{)}^{1/2}\) (Ennis-King & Paterson 2005). For a CO2 diffusivity in brine of 1.9 × 10–9 m2/s and an elapsed time of 3600 s, the thickness of a CO2 laden layer is about 5 mm. Figure 2 indicates, however, that only very short times are controlled by diffusion across the gas-brine interface. Times up to about 100 min follow the dispersive limit (dotted line in Fig. 2) supporting the inclusion of dispersion in Eq. (5).
Summary of the dissolution of CO2 into the aqueous phase for 5 repeat experiments. The fraction refers to the total mass dissolved over the lifetime of an experiment. The diffusive limit is computed using \({\mathcal{D}}_{cb}\) = 1.9 × 10–9 m2/s and Eq. (8) from (Hassanzadeh et al. 2007). Dispersion coefficient is found as \({D}_{c}/{\mathcal{D}}_{c}=10\) and the dispersive limit is computed similar to the diffusive limit. Note linear behavior during early convective mixing between roughly 100 and 300 min. The inset indicates that the experiments deviate from diffusive transport at times less than 10 min. Experimental time resolution is ∆t = 5 min
Consistent with linear stability analysis of miscible displacement (Elenius and Johannsen 2012), Fig. 1 illustrates early onset of instability, whereas Fig. 2 shows that this initial unstable regime follows t1/2 scaling until about 150 min. The fully unstable system then emerges, mixing evolves, the rate of mass transfer increases, and the scaling of mass in solution transitions to t (Hassanzadeh et al. 2007) as illustrated by the straight line in Fig. 2 until roughly 400 min.
5.1 Onset of Fingering
Visually, the onset time of unstable miscible fingers between the CO2 saturated and unsaturated brine is relatively rapid following the establishment of the region saturated with free-phase CO2. Figure 1a shows potential evidence of fingers after 34 min of CO2 injection in the FluidFlower. By this time a distinct and widespread gas phase has formed along with a narrow region of CO2-saturated brine beneath the gas zone.
A variety of analytical and numerical treatments of the instability of CO2-laden brine layers and subsequent convective mixing of the brine zone beneath the gas cap are available. Notably, much of the analysis in the literature assumes the rapid accumulation of a quiescent zone of free-phase CO2 atop brine followed by dissolution and gravitational instability (Ennis-King and Paterson 2005; Hassanzadeh et al. 2007; Riaz et al. 2006). On the other hand, the results summarized in Fig. 1 support the notion that the fingering begins during active injection and the capillary fringe beneath the gas cap interacts with the CO2-laden brine in the diffusive boundary layer. This interaction is predicted to reduce the time required for the onset of finger formation by up to a factor of 5 (Elenius et al. 2012).
Elenius et al. (2012) propose that the onset time, tf, for convective mixing of the brine-filled zone lies within a range incorporating time scales for horizontal and vertical flow components as
Due to the relatively fast advance of the gas/liquid transition zone in the FluidFlower and the absence of a remarkable period of time dominated by diffusion in Fig. 2, we substitute dispersion coefficient for diffusion coefficient within the inequality in Eq. (11). Additionally, the vertical permeability is obtained as the product of kR and kV/kH from Table 2.
Taking values from Table 2 and setting the difference in density to 3.5 kg/m3 (Efika et al. 2016), we obtain prediction of onset time that ranges from 1 to 6 min for the FluidFlower using Eq. (11). Other predictions available from the literature are also summarized in Table 5. Experimentally, the onset time for fingering in the FluidFlower depends on the time required to build the gas-filled region in the top of the storage zone. Onset time is found by plotting the position of fingers versus time and extrapolating the position to zero. Then, the time needed for CO2 to flow from the injection point and to accumulate in the volume above the finger is subtracted to obtain the onset time. Onset times for fingering in the FluidFlower are thus found to be about 28 min. This is in order of magnitude agreement with Fig. 1a.
Experiments can also be compared to predictions of the critical wavelength, λf, of instabilities that grow into fingers. Similar to onset time, Elenius et al. (2012) suggest that realistic cases for λf are bounded as
where diffusion has been substituted by dispersion coefficient. With the same input as that used to evaluate Eq. (11), λf ranges from 1.6 to 2.0 cm in Table 5. Experimental images are analyzed as described by Nordbotten et al. (2023). Inspection of experimental results from the FluidFlower in Fig. 3 indicates that significant merging of fingers does not occur at relatively short times. Hence, the wavelength of macroscopic-dimension fingers at these times likely corresponds to the critical wavelength. Figure 3 indicates roughly 31 fingers below the gas cap of the storage zone in the box outlined in gray. This zone is about 1.5 m in length. Hence, the experimental wavelength of the fingers is about 4.8 ± 0.3 cm on average.
Fingers during experiments using image analysis: (a) example labeling of fingers in a zone that is 1.5 m in length and (b) evolution of the number of fingers for 5 repeat experiments. The maximum number of fingers is found at times from roughly 160–250 min. During this time, the average number of fingers over the 5 experiments is 31 with a standard deviation of 2
The ability of Eqs. (11) and (12) to reflect the dynamics in the FluidFlower gives us some confidence to apply them to the storage formation examples (Table 5). Generally, critical wavelengths range from 1 to 10's of m, whereas onset times range from less than a year for Sleipner conditions up to 10's of thousands of years for In Salah conditions due to small kV.
5.2 End of Early Convective Mixing
The formation of fingers marks the start of convective mixing and mixing stratifies the density gradients in the brine. This stratification of density diminishes mixing and eventually leads to the reduction or gradual elimination of convection cells. Linear stability analysis is no longer applicable. Analysis of numerical simulations showed that the end of the early convective mixing period is expressed as Hassanzadeh et al. (2007)
where diffusion has again been substituted with dispersion coefficient. With the input previously used in Eqs. (11) and (12) for the FluidFlower, Eq. (13) predicts the end of the period of convective mixing driven by fingering to be 570 min in reasonable order of magnitude agreement with results in Figs. 1c and 2. In Fig. 2, the end of early mixing is gauged by the slope of the dissolved mass curves deviating from near constant and decreasing (Hassanzadeh et al. 2007). Note that the applicability of Eq. (13) was checked as the range of suitability is 80 < Ra < 2000 where Ra (\(=\Delta \rho gkH/\phi {\mu }_{b}{D}_{cb}\)) is the Rayleigh number. For FluidFlower conditions, Ra equals 453.
6 Discussion
The large formation heights raise the question of the neglect of vertical temperature gradients in the storage formation. Hence, we briefly revisit the first simplification. The vertical temperature gradient in a storage formation might be 0.03 °C/m. In the case of Sleipner (Table 2), the temperature difference is 6 °C across the storage formation. At 38 °C and 9.5 MPa, CO2 viscosity is 4.59 × 10–5 Pa-s and density is 597 kg/m3, whereas at 44 °C and 9.5 MPa the viscosity and density are 3.51 × 10–5 Pa-s and 468 kg/m3, respectively. These are changes of about 12% relative to values at 41 °C. Neglecting the variation in fluid properties due to temperature gradient in simplification 1 does not appear to be of primary importance for our analysis.
The physical processes considered here included flow driven by pressure gradient, flow driven by gravity, gas-to-brine interphase mass transfer controlled by diffusion, dispersive transport within brine, and convective mixing of dense CO2-laden brine with the original aqueous phase. Visualization of experiments teaches that flow is the dominant mass transfer mechanism of both the gas and brine phases, and this observation guided the scaling analysis. Figure 4 summarizes visually the magnitudes of the coefficients in Eq. (5) using characteristic parameters. It illustrates that all coefficients are at least an order of magnitude less than 1, implying that flow is the dominant process at both laboratory and field scale under the conditions studied. The impact of buoyancy-driven flow is most significant in the permeable Northern Lights and Sleipner example field cases.
Additionally, Fig. 5 presents the difference in the relative importance of processes between the FluidFlower and the storage formations as the ratio of the scaling groups that emerged from Eq. (5) when evaluated with reference values from Tables 2 and 4. Pressure-driven flow in the z-direction has a ratio of 1 because the ordering process proceeded from this choice. Figure 5 shows that the x-direction pressure gradient and intraphase mass transfer are relatively greater in the FluidFlower compared to storage formations. On the other hand, gravity-driven flow is relatively smaller in the FluidFlower. These predictions result in large part from the significant heights and lengths of the storage formation in comparison to the FluidFlower. For example, intraphase mass transfer in the FluidFlower is predicted to be about 100 times greater than characteristic Sleipner conditions largely due to the substantial sand thickness at Sleipner.
Ratio of scaling groups evaluated using characteristic values for FluidFlower and storage formation conditions. The ratios illustrate the relative time scales between the FluidFlower and the storage formation not the relative importance of each process. Pressure-driven flow in the z-direction has a scaling of 1 for all cases and allows estimation of storage formation time scales from FluidFlower results
Convective mixing of CO2-laden brine with original brine is a significant mass transfer mechanism during CO2 storage (Lindeberg and Wessel-Berg 1997; Weir et al. 1995). Application of results from linear stability analysis to FluidFlower conditions produced order of magnitude agreement for the critical wavelength of instabilities of about 4.8 cm. The critical time for the onset of instabilities, however, is predicted to differ from what is found experimentally. The physical situation in the FluidFlower does not agree with the theoretical analysis because the FluidFlower requires some time to accumulate a gas-phase region and that region subsequently grows laterally and vertically in time. Visual inspection suggests that the capillary transition zone at the base of the gas-phase region and the advancing gas/liquid interface plays a role in relatively short onset times for convective mixing. Overall, fingers in the FluidFlower establish themselves rapidly. The net effect appears to be that CO2 goes into solution in brine and mixes with the original pore waters very rapidly in the FluidFlower. This aspect warrants further investigation.
Alternate expressions for the critical onset time for fingering and the critical wavelength of fingers, Eqs. (10) and (11), were also explored. These expressions tend to produce estimates of the critical time for the onset of fingering that are substantially greater than the analysis of Elenius et al. (2012) and somewhat closer to the experimental observations. On the other hand, estimates of the critical wavelength were all on the order of cm and in agreement with FluidFlower observations.
Interestingly, the various predictions for the onset of time of convective mixing in the case of In Salah are on the order of thousand to millions of years, but the Northern Lights and Sleipner cases were years to 10's of years. In these latter cases, convective mixing of CO2-saturated brine begins during injection. Accordingly, CO2 goes into solution relatively quickly and convective mixing contributes to rapid downward migration of CO2 during the injection period. This aspect improves storage security.
The reactions of dissolved CO2 to create carbonate minerals and the dissolution of minerals were not analyzed here. The timescale of mineral trapping is 1000's to 10,000's of years in sedimentary sandstone formations that do not contain relatively abundant and soluble silicate minerals (Audigane et al. 2007; Zhang and DePaolo 2017). Such solubilization releases calcium, magnesium, and iron species that may combine with CO2. Hence, the formation of carbonate minerals is expected to contribute little to the sequestration of CO2 during the period of active injection and thereafter for quite some time in the example formations used here. Formations with the potential for rapid carbon mineralization contain mafic and ultramafic igneous or metamorphic rocks, such as basalt. Incorporation of mineralization driven by low pH brine into the scaling analysis represents a significant extension due to reaction network complexity. This is left as a potential topic for future work.
7 Summary and Conclusion
A mass balance incorporating rate-dependent mass transfer for CO2 interaction with brine under immiscible conditions was analyzed to compare laboratory conditions at ambient temperature and pressures with storage formations at temperature ranging from 41 to 95 °C and pressures up to 29 MPa. The period of interest was active CO2 injection operations. Comparison of the dimensionless groups developed by an ordering analysis showed that conditions in the FluidFlower and storage formations are largely dominated by transport driven by the imposed pressure gradient. The contribution of gravity to convective transport is somewhat greater in storage formations as compared to the FluidFlower. Results indicate that free-phase CO2 movement, saturation of brine beneath the gas zone with CO2, and downward migration of CO2-laden brine through CO2-free brine, as seen in the FluidFlower during injection, are relevant to storage formations where similar mass transfer and physical processes are expected. Accordingly, relatively straightforward scaling of time using characteristic quantities (Eq. 8) is possible between the FluidFlower and storage formation conditions.
Significant convective mixing of CO2 that has dissolved into formation brine with CO2-free brine is found in the FluidFlower. The onset time for downward flow of CO2 is only a fraction of the total duration of CO2 injection. Hence, the condition of quiescent fluids prior to convective mixing of brine, as assumed in many theoretical analyses, is not met in the FluidFlower. Application of predictions for onset times to representative storage formation conditions likewise teaches that the onset time for fingering is significantly less than the duration of CO2 injection. The implications of this observation include that mixing of CO2 with brine and the subsequent settling due to gravity may be more rapid than some prior predictions. More rapid mixing is a favorable outcome enhancing CO2 storage security.
Abbreviations
- a i :
-
Interfacial area
- B :
-
Constant in Eq. (8)
- c :
-
Constant in Eq. (10)
- D :
-
Dispersion
- Ɗ :
-
Diffusivity
- g :
-
Acceleration due to gravity
- h :
-
Henry's law constant
- H :
-
Characteristic vertical dimension
- J i :
-
Diffusive flux of component i
- k :
-
Absolute permeability
- K :
-
Mass transfer coefficient
- k r :
-
Relative permeability
- L :
-
Characteristic horizontal length
- p :
-
Pressure
- q :
-
Injection/production rate source sink term
- S :
-
Saturation
- t :
-
Time
- T :
-
Absolute temperature
- u :
-
Darcy velocity
- v :
-
Interstitial velocity
- x :
-
Horizontal distance
- z :
-
Vertical distance
- ϕ :
-
Porosity
- λ :
-
Wavelength
- µ :
-
Viscosity
- \({\varrho }\) :
-
Mass density
- ν :
-
Kinematic viscosity
- ρ :
-
Molar density
- τ :
-
Tortuosity
- χ :
-
Mole fraction
- β :
-
Phase
- b :
-
Aqueous phase
- c :
-
CO2 chemical component
- D :
-
Dimensionless
- f :
-
Fingering
- g :
-
CO2-rich phase
- H :
-
Horizontal direction
- i :
-
Component
- R :
-
Reference or characteristic value
- V :
-
Vertical direction
- w :
-
Water chemical components
References
Audigane, P., Gaus, I., Czernichowski-Lauriol, I., Pruess, K., Xu, T.: Two-dimensional reactive transport modeling of CO2 injection in a saline aquifer at the Sleipner site, North Sea. Am. J. Sci. 307(7), 974–1008 (2007). https://doi.org/10.2475/07.2007.02
Barenblatt, G.I.: Scaling, self-similarity, and intermediate asymptotics: dimensional analysis and intermediate asymptotics, 1st edn. Cambridge University Press (1996). https://doi.org/10.1017/CBO9781107050242
Basu, A., Islam, M.R.: Scaling up of chemical injection experiments. Pet. Sci. Technol. 27(7), 654–665 (2009). https://doi.org/10.1080/10916460802105534
Bissell, R.C., Vasco, D.W., Atbi, M., Hamdani, M., Okwelegbe, M., Goldwater, M.H.: A full field simulation of the in Salah gas production and CO2 storage project using a coupled geo-mechanical and thermal fluid flow simulator. Energy Procedia 4, 3290–3297 (2011). https://doi.org/10.1016/j.egypro.2011.02.249
Both, J., Storvik, E., Nordbotten, J. M., & Benali, B. (2023a). DarSIA v1.0 (v1.0) [Computer software]. Zenodo. https://doi.org/10.5281/ZENODO.7515016
Both, J.W., Benali, B., Folkvord, O., Haugen, M., Storvik, E., Fernø, M., Nordbotten, J.M.: Image Analysis of the International FluidFlower Benchmark Study Dataset (v1.0) [Computer software]. Zenodo (2013b). https://doi.org/10.5281/ZENODO.7515038
Cadogan, S.P., Maitland, G.C., Trusler, J.P.M.: Diffusion coefficients of CO2 and N2 in water at temperatures between 298.15 K and 423.15 K at pressures up to 45 MPa. J. Chem. Eng. Data 59(2), 519–525 (2014). https://doi.org/10.1021/je401008s
Callas, C., Kovscek, A.R., Benson, S.M.: Assessing the impact of dip angle on carbon storage in saline reservoirs to aid site selection. Int. J. Greenhouse Gas Control 129, 103966 (2023). https://doi.org/10.1016/j.ijggc.2023.103966
Chadwick, R.A.: Offshore CO2 storage: Sleipner natural gas field beneath the North Sea. In: Geological Storage of Carbon Dioxide (CO2), pp. 227–253e. Elsevier (2013). https://doi.org/10.1533/9780857097279.3.227
Chadwick, R.A., Williams, G.A., Williams, J.D.O., Noy, D.J.: Measuring pressure performance of a large saline aquifer during industrial-scale CO2 injection: the Utsira Sand, Norwegian North Sea. Int. J. Greenhouse Gas Control 10, 374–388 (2012). https://doi.org/10.1016/j.ijggc.2012.06.022
Denn, M.: Process fluid mechanics. Prentice-Hall (1980)
Efika, E.C., Hoballah, R., Li, X., May, E.F., Nania, M., Sanchez-Vicente, Y., Martin Trusler, J.P.: Saturated phase densities of (CO2 + H2O) at temperatures from (293 to 450) K and pressures up to 64 MPa. J. Chem. Thermodyn. 93, 347–359 (2016). https://doi.org/10.1016/j.jct.2015.06.034
Eikehaug, K., Bang Larsen, E., Haugen, M., Folkvord, O., Benali, B., Both, J., Nordbotten, J.M., Fernø, M.: The International FluidFlower benchmark study dataset (0.1). Zenodo (2023). https://doi.org/10.5281/ZENODO.7510589
Eikehaug, K., Haugen, M., Folkvord, O., Benali, B., Larsen, E.B., Tinkova, A., Rotevatn, A., Nordbotten, J.M., Fernø, M.A.: Engineering Meter-scale Porous Media Flow Experiments for Quantitative Studies of Geological Carbon Sequestration. Transp Porous Med (2024). https://doi.org/10.1007/s11242-023-02025-0
Elenius, M.T., Johannsen, K.: On the time scales of nonlinear instability in miscible displacement porous media flow. Comput. Geosci. 16(4), 901–911 (2012). https://doi.org/10.1007/s10596-012-9294-2
Elenius, M.T., Nordbotten, J.M., Kalisch, H.: Effects of a capillary transition zone on the stability of a diffusive boundary layer. IMA J. Appl. Math. 77(6), 771–787 (2012). https://doi.org/10.1093/imamat/hxs054
Enick, R.M., Klara, S.M.: CO2 solubility in water and brine under reservoir conditions. Chem. Eng. Commun. 90(1), 23–33 (1990). https://doi.org/10.1080/00986449008940574
Ennis-King, J., Paterson, L.: Role of convective mixing in the long-term storage of carbon dioxide in deep saline formations. SPE J. 10(03), 349–356 (2005). https://doi.org/10.2118/84344-PA
Erfani, H., Babaei, M., Berg, C.F., Niasar, V.: Scaling CO2 convection in confined aquifers: Effects of dispersion, permeability anisotropy and geochemistry. Adv. Water Resour. 164, 104191 (2022). https://doi.org/10.1016/j.advwatres.2022.104191
Fernø, M.A., Haugen, M., Eikehaug, K., Folkvord, O., Benali, B., Both, J., Storvik, E., Nixon, C., Gawthorpe, R., Nordbotten, J.: Room-scale CO2 injections in a physical reservoir model with faults. Transp Porous Med (2023). https://doi.org/10.1007/s11242-023-02013-4
Flemisch, B., Nordbotten, J.M., Fernø, M., Juanes, R., Both, J.W., Class, H., Delshad, M., Doster, F., Ennis-King, J., Franc, J., Geiger, S., Gläser, D., Green, C., Gunning, J., Hajibeygi, H., Jackson, S.J., Jammoul, M., Karra, S., Li, J., Zhang, Z.: The FluidFlower validation benchmark study for the storage of CO2. Transp. Porous Media (2023). https://doi.org/10.1007/s11242-023-01977-7
Golding, M.J., Huppert, H.E., Neufeld, J.A.: Two-phase gravity currents resulting from the release of a fixed volume of fluid in a porous medium. J. Fluid Mech. 832, 550–577 (2017). https://doi.org/10.1017/jfm.2017.437
Grattoni, C.A., Jing, X.D., Dawe, R.A.: Dimensionless groups for three-phase gravity drainage flow in porous media. J. Petrol. Sci. Eng. 29(1), 53–65 (2001). https://doi.org/10.1016/S0920-4105(00)00090-5
Green, C., Jackson, S.J., Gunning, J., Wilkins, A., Ennis-King, J.: Modelling the FluidFlower: insights from characterisation and numerical predictions. Transp. Porous Media (2023). https://doi.org/10.1007/s11242-023-01969-7
Gupta, A.K., Bryant, S.L.: Analytical models to select an effective saline reservoir for CO2 storage. All Days (2010). https://doi.org/10.2118/134762-MS
Hassanzadeh, H., Pooladi-Darvish, M., Keith, D.W.: Scaling behavior of convective mixing, with application to geological storage of CO2. AIChE J. 53(5), 1121–1131 (2007). https://doi.org/10.1002/aic.11157
Haugen, M., Saló-Salgado, L., Eikehaug, K., Benali, B., Both, J., Storvik, E., Folkvord, O., Juanes, R., Nordbotten, J.M., & Fernø, M.A.: Physical variability in meter-scale laboratory CO2 injections in faulted geometries. Transport in Porous Media, in press (2024) Preprint:https://arxiv.org/ftp/arxiv/papers/2301/2301.07347.pdf
Hesse, M.A., Tchelepi, H.A., Orr, F.M.: Scaling analysis of the migration of CO2 in saline aquifers. All Days (2006). https://doi.org/10.2118/102796-MS
Hesse, M.A., Orr, F.M., Tchelepi, H.A.: Gravity currents with residual trapping. J. Fluid Mech. 611, 35–60 (2008). https://doi.org/10.1017/S002211200800219X
Hidalgo, J.J., Carrera, J.: Effect of dispersion on the onset of convection during CO2 sequestration. J. Fluid Mech. 640, 441–452 (2009). https://doi.org/10.1017/S0022112009991480
IEA: CCUS in clean energy transitions. IEA. IEA (2020), CCUS in Clean Energy Transition. (2020), https://www.iea.org/reports/ccus-in-clean-energy-transitions
Islam, M.R., Ali, S.M.: New scaling criteria for chemical flooding experiments. J. Can. Petrol. Technol. (1990). https://doi.org/10.2118/90-01-02
Islam, M.R., Ali, S.M.F.: New scaling criteria for in-situ combustion experiments. J. Petrol. Sci. Eng. 6(4), 367–379 (1992). https://doi.org/10.1016/0920-4105(92)90063-7
Jammoul, M., Delshad, M., Wheeler, M.F.: Numerical modeling of CO$$_2$$ storage: applications to the FluidFlower experimental setup. Transp. Porous Media (2023). https://doi.org/10.1007/s11242-023-01996-4
Jha, R.K., Bryant, S.L., Lake, L.W.: Effect of diffusion on dispersion. SPE J. 16(01), 65–77 (2011). https://doi.org/10.2118/115961-PA
Juanes, R., MacMinn, C.W.: Upscaling of capillary trapping under gravity override: application to CO2 sequestration in aquifers. All Days (2008). https://doi.org/10.2118/113496-MS
Juanes, R., MacMinn, C.W., Szulczewski, M.L.: The footprint of the CO2 plume during carbon dioxide storage in saline aquifers: storage efficiency for capillary trapping at the basin scale. Transp. Porous Media 82(1), 19–30 (2010). https://doi.org/10.1007/s11242-009-9420-3
Keilegavlen, E., Fonn, E., Johannessen, K., Eikehaug, K., Both, J., Fernø, M., Kvamsdal, T., Rasheed, A., Nordbotten, J.M.: PoroTwin: a digital twin for a FluidFlower rig. Transp. Porous Med (2023). https://doi.org/10.1007/s11242-023-01992-8
Kimber, K.D., Ali, S.M.F.: Verification of scaling approaches for steam injection experiments. J. Can. Petrol. Technol. (1989). https://doi.org/10.2118/89-01-03
Kneafsey, T.J., Pruess, K.: Laboratory flow experiments for visualizing carbon dioxide-induced, density-driven brine convection. Transp. Porous Media 82(1), 123–139 (2010). https://doi.org/10.1007/s11242-009-9482-2
Lake, L.W., Johns, R., Rossen, W., Pope, G.: Fundamentals of enhanced oil recovery, 2nd edn. Society of Petroleum Engineers (2014)
Lange, N.A.: In: J. G. Speight (Eds.) Lange’s Handbook of Chemistry. 17 edition. McGraw-Hill Education (2017)
Lewis, W.K., Whitman, W.G.: Principles of gas absorption. Ind. Eng. Chem. 16(12), 1215–1220 (1924). https://doi.org/10.1021/ie50180a002
Lindeberg, E., Wessel-Berg, D.: Vertical convection in an aquifer column under a gas cap of CO2. Energy Convers. Manage. 38, S229–S234 (1997). https://doi.org/10.1016/S0196-8904(96)00274-9
Lozada, D., Ali, S.M.F.: New sets of scaling criteria for partial equilibrium immiscible carbon dioxide drive. Annu Techn Meet (1987). https://doi.org/10.2118/87-38-23
MacMinn, C.W., Szulczewski, M.L., Juanes, R.: CO2 migration in saline aquifers. Part 2. Capillary and solubility trapping. J. Fluid Mech. 688, 321–351 (2011). https://doi.org/10.1017/jfm.2011.379
Marashi, S.V.: Northern lights project: aurora model investigation with sensitivity studies and using different simulation methods. Norwegian University of Science and Technology (NTNU) (2021)
Martin, J.M., Combarnous, M., Charpentier, J.C.: Mass transfer and phase distribution for two-phase flow through porous media. Chem. Eng. Commun. 10(4–5), 269–281 (1981). https://doi.org/10.1080/00986448108910939
Nordbotten, J.M., Benali, B., Both, J.W., Brattekås, B., Storvik, E., Fernø, M.A.: DarSIA: an open-source python toolbox for two-scale image processing of dynamics in porous media. Transp. Porous Media (2023). https://doi.org/10.1007/s11242-023-02000-9
Ogata, A., Banks, R.F.: A solution of the differential equation of longitudinal dispersion in porous media: Fluid movement in earth materials (411-A; US Geological Survey Professional Paper, p. 6). USGS (1961)
Pau, G.S.H., Bell, J.B., Pruess, K., Almgren, A.S., Lijewski, M.J., Zhang, K.: High-resolution simulation and characterization of density-driven flow in CO2 storage in saline aquifers. Adv. Water Resour. 33(4), 443–455 (2010). https://doi.org/10.1016/j.advwatres.2010.01.009
Prausnitz, J.M., Lichtenthaler, R.N., de Azevedo, E.G.: Molecular thermodynamics of fluid-phase equilibria, 3rd edn. Prentice Hall PTR (1999)
Riaz, A., Hesse, M., Tchelepi, H.A., Orr, F.M.: Onset of convection in a gravitationally unstable diffusive boundary layer in porous media. J. Fluid Mech. 548(1), 87 (2006). https://doi.org/10.1017/S0022112005007494
Ringrose, P., Atbi, M., Mason, D., Espinassous, M., Myhrer, Ø., Iding, M., Mathieson, A., Wright, I.: Plume development around well KB-502 at the In Salah CO2 storage site. First Break (2009). https://doi.org/10.3997/1365-2397.27.1295.28744
Saló-Salgado, L., Haugen, M., Eikehaug, K., Fernø, M., Nordbotten, J.M., Juanes, R.: Direct comparison of numerical simulations and experiments of CO2 injection and migration in geologic media: value of local data and forecasting capability. Transp. Porous Media (2023). https://doi.org/10.1007/s11242-023-01972-y
Sharma, A.P., Rao, D.N.: Scaled physical model experiments to characterize the gas-assisted gravity drainage EOR process. All Days (2008). https://doi.org/10.2118/113424-MS
Sundaram, N.S., Islam, M.R.: Scaled model studies of petroleum contaminant removal from soils using surfactant solutions. J. Hazard. Mater. 38(1), 89–103 (1994). https://doi.org/10.1016/0304-3894(93)E0135-O
Tamimi, A., Rinker, E.B., Sandall, O.C.: Diffusion coefficients for hydrogen sulfide, carbon dioxide, and nitrous oxide in water over the temperature range 293–368 K. J. Chem. Eng. Data 39(2), 330–332 (1994). https://doi.org/10.1021/je00014a031
Trevisan, L., Pini, R., Cihan, A., Birkholzer, J.T., Zhou, Q., González-Nicolás, A., Illangasekare, T.H.: Imaging and quantification of spreading and trapping of carbon dioxide in saline aquifers using meter-scale laboratory experiments: experimental analysis of CO2 migration. Water Resour. Res. 53(1), 485–502 (2017). https://doi.org/10.1002/2016WR019749
van Lookeren, J.: Calculation methods for linear and radial steam flow in oil reservoirs. Soc. Petrol. Eng. J. 23(03), 427–439 (1983). https://doi.org/10.2118/6788-PA
Vella, D., Huppert, H.E.: Gravity currents in a porous medium at an inclined plane. J. Fluid Mech. 555, 353 (2006). https://doi.org/10.1017/S0022112006009578
Wang, Y., Zhang, Z., Vuik, C., Hajibeygi, H.: Simulation of CO2 storage using a parameterization method for essential trapping physics fluidflower benchmark study (2023). Doi: https://doi.org/10.48550/ARXIV.2301.08004
Wapperom, M., Tian, X., Novikov, A., Voskov, D.: FluidFlower benchmark: lessons learned from the perspective of subsurface simulation. Transp. Porous Media (2023). https://doi.org/10.1007/s11242-023-01984-8
Weir, G.J., White, S.P., Kissling, W.M.: Reservoir storage and containment of greenhouse gases. Energy Convers. Manage. 36(6–9), 531–534 (1995). https://doi.org/10.1016/0196-8904(95)00060-Q
Werner, M., Sutter, D., Krättli, A., Lafci, Ö., Mutschler, R., Oehler, P., Winkler, J., Mazzotti, M.: A physical model for geological CO2 storage: replacing misconceptions by visual explanation. Int. J. Greenhouse Gas Control 25, 42–53 (2014). https://doi.org/10.1016/j.ijggc.2014.03.014
Yortsos, Y.C.: A theoretical analysis of vertical flow equilibrium. Transp. Porous Media 18(2), 107–129 (1995). https://doi.org/10.1007/BF01064674
Zhang, S., DePaolo, D.J.: Rates of CO2 mineralization in geological carbon storage. Acc. Chem. Res. 50(9), 2075–2084 (2017). https://doi.org/10.1021/acs.accounts.7b00334
Acknowledgements
We thank B. Benali and J.W. Both for assistance in analyzing FluidFlower results.
Funding
ARK acknowledges the support of the University of Bergen for a sabbatical visit, the Stanford University Energy Transition Research Institute (SUETRI-A), and the Stanford Center for Carbon Storage (SCCS). JMN and MAF acknowledge the support from Centre for Sustainable Subsurface Resources (Research Council of Norway grant number 331841).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no known competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix A
Appendix A
1.1 Mass Transfer Resistance
This appendix develops the two-film model for mass transfer resistance that is used to find overall mass transfer resistance following the ideas of Lewis and Whitman (1924). Figure
6 sketches bulk fluid phases with an interfacial region. The interface is marked as a black dashed line. The overall mass transfer resistance describes transfer from the g to the b phase. There are stagnant films with unequal dimensions on each side of the interface. A Henry's law relation is written as
to describe the equilibrium solubility of CO2 in the brine phase. It is assumed that only the interface is at equilibrium and so describable by Henry's law. The flux across film-1 is equal to the flux across film-2 and written as
where the subscript i denotes conditions at the interface, Kg and Kb are mass transfer coefficients for the respective films, and it is clear that Kg and Kb have different units.
To proceed, Eq. (14) is substituted into the expression for mass transfer in Eq. (15) and the result solved for pci to obtain
Equation (17) is then substituted into the expression for mass transfer in the CO2-rich phase in Eq. (16) and solved for the flux as
Equation (18) describes the flux of CO2 from phase g to b and is rewritten as
where the overall mass transfer coefficient is found as
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kovscek, A.R., Nordbotten, J.M. & Fernø, M.A. Scaling Up FluidFlower Results for Carbon Dioxide Storage in Geological Media. Transp Porous Med 151, 975–1002 (2024). https://doi.org/10.1007/s11242-023-02046-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-023-02046-9