Abstract
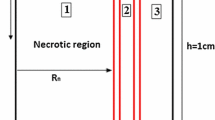
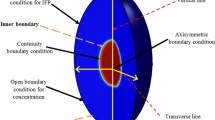
Chemotherapy as one of the most utilized cancerous tumor treatment methods introduces undesired side effects due to penetrating toxic drugs into the healthy organs. Delivery of anticancer therapeutic agents to solid tumors is also problematic. The purpose of current study is to investigate the penetration of magnetic drug carriers (MDCs) within the cancerous solid tumor tissue under the influence of external magnet. Capillary wall and tumor tissue is modeled as a saturated porous media. In order to solve the coupled governing equations, mass, momentum and concentration, an in-house finite volume-based code is developed and utilized. Results show the penetration of MDCs into the tumor in the absence of magnetic field is minimal and is limited to the surface of the tumor. On the other hand, under the influence of external magnet the penetration of MDCs within the tumor increases exponentially. They also penetrate deep into the tumor and cover the entire tumor which increase the effectivity and decrease the side effect of the treatment.













Similar content being viewed by others
Abbreviations
- \(B_{0}\) :
-
Magnetic flux density of external magnet at its surface
- \(C_{0}\) :
-
MDCs concentration at inlet
- C :
-
MDCs dimensionless concentration
- D :
-
MDCs diffusion coefficient
- \(D_{\infty }\) :
-
diffusion coefficient of particle in unbounded fluid
- \(D_{\mathrm{blood}}\) :
-
MDCs diffusion coefficient in the blood
- \(D_{\mathrm{B}}\) :
-
Brownian diffusion coefficient of MDCs
- \(D_{\mathrm{S}}\) :
-
Scattering diffusion coefficient of MDCs
- \(D_{\mathrm{Endo}}\) :
-
MDCs diffusion coefficient in the Endothelium layer
- \(D_{\mathrm{Tissue}}\) :
-
MDCs diffusion coefficient in the Tumor tissue
- E :
-
Uptake term
- \(\mathbf{F}_{1}\) :
-
Magnetic force acting upon a single MDC
- \(F_{\mathrm{x}}\) :
-
Horizontal magnetic body force
- \(F_{\mathrm{y}}\) :
-
Vertical magnetic body force
- G :
-
Generation term
- H :
-
Magnetic field intensity
- J :
-
Hydrodynamic coefficient
- P :
-
Pressure
- \(\textit{Re}\) :
-
Reynolds number
- \(r_{\mathrm{mag}}\) :
-
External magnet radius
- S :
-
Steric coefficient
- \(U_{\mathrm{in}}\) :
-
Inlet blood velocity
- u :
-
Horizontal blood velocity
- v :
-
Vertical blood velocity
- \(\vec {\mathbf{v}}\) :
-
Blood velocity vector
- \(\vec {\mathbf{v}}_{\mathrm{MDC}}\) :
-
MDCs velocity vector
- \(\vec {\mathbf{v}}_{\mathrm{relative}}\) :
-
Relative velocity of MDCs to blood
- \(x_{\mathrm{mag}}\) :
-
Horizontal position of external magnet
- \(y_{\mathrm{mag}}\) :
-
Vertical position of external magnet
- z :
-
Distance between external magnet and tumor
- \(\rho \) :
-
Blood density
- \(\varepsilon \) :
-
Porosity
- \(\mu \) :
-
Blood viscosity
- \(\mu _{\mathrm{plasma}}\) :
-
Plasma viscosity
- \(\mu _{0}\) :
-
Magnetic permeability of vacuum
- \(\lambda _{\mathrm{g}}\) :
-
Geometrical tortuosity
- \(\chi \) :
-
Magnetic susceptibility of the MNPs
- \(\tau _{\mathrm{p}}\) :
-
Particle response time
- \(\forall _{\mathrm{MNP}}\) :
-
Total volume of MNPs in single MDC
- \(\forall _{\mathrm{MDC}}\) :
-
Volume of a single MDC
References
Ai, L., Vafai, K.: A coupling model for macromolecule transport in a stenosed arterial wall. Int. J. Heat Mass Transf. 49, 1568–1591 (2006)
Berthier, S.: Microfluidics for Biotechnology. Artech House Inc, Boston (2006)
Bhujwalla, Z., McCoy, C., Glickson, J., GiIfies, R., Stubbs, M.: Estimations of intra- and extracellular volume and pH by 31P magnetic resonance spectroscopy: effect of therapy on RIF-1 tumours. Br. J. Cancer 78(5), 606–611 (1998)
Cao, Q., Han, X., Li, L.: Numerical analysis of magnetic nanoparticle transport in microfluidic systems under influence of permanent magnets. J. Phys. D: Appl. Phys. 45(46), 465001 (2012)
Dreher, M.R., Liu, W., Michelich, C.R., Dewhirst, M.W., Yuan, F., Chilkoti, A.: Tumor vascular permeability, accumulation, and penetration of macromolecular drug carriers. J. Natl. Cancer Inst. 98(5), 335–344 (2006)
Fournier, R.L.: Basic Transport Phenomena in Biomedical Engineering. CRC Press, New York (2011)
Freitas Jr., R.A.: Nanomedicine (Vol. I: Basic copabilities). Bioscience, George town (1999)
Graff, B., Bjørnæs, I., Rofstad, E.: Macromolecule uptake in human melanoma xenografts: relationships to blood supply, vascular density, microvessel permeability and extracellular volume fraction. Eur. J. Cancer 36, 1433–1440 (2000)
Grief, A., Richardson, G.: Mathematical modelling of magnetically targeted drug delivery. J. Magn. Magn. Mater. 293, 455–463 (2005)
Habibi, M.R., Ghasemi, M.: Numerical study of magnetic nanoparticles concentration in biofluid (blood) under influence of high gradient magnetic field. J. Magn. Magn. Mater. 323, 32–38 (2011)
Habibi, M.R., Ghassemi, M., Hamedi, M.H.: Analysis of high gradient magnetic field effects on distribution of nanoparticles injected into pulsatile blood stream. J. Magn. Magn. Mater. 324, 1473–1482 (2012)
Hashizume, H., Baluk, P., Morikawa, S., McLean, J.W., Thurston, G., Roberge, S., Rakesh, K., McDonald, D.M.: Openings between defective endothelial cells explain tumor vessel leakiness. Am. J. Pathol. 156, 1363–1380 (2000)
Hobbs, S.K., Monsky, W.L., Yuan, F., Roberts, W.G., Griffith, L., Torchilin, V.P., Jain, R.K.: Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl. Acad. Sci. USA 95, 4607–4612 (1998)
Jain, R.K.: Transport of molecules in the tumor interstitium: a review. Cancer Res. 47, 3039–3051 (1987)
Jain, R.K., Baxter, L.T.: Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res. 48, 7022–7032 (1988)
Jain, R.K., Stylianopoulos, T.: Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7, 653–664 (2010)
Késmárky, G., Kenyeres, P., Rábai, M., Tóth, K.: Plasma viscosity: a forgetten variable. Clin. Heortheol. Microcirc. 39, 243–246 (2008)
Keangin, P., Vafai, K., Rattanadecho, P.: Electromagnetic field effects on biological materials. Int. J. Heat Mass Transf. 65, 389–399 (2013)
Khanafer, K., Vafai, K.: The role of porous media in biomedical engineering as related to magnetic resonance imaging and drug delivery. Heat Mass Transf. 42, 939–953 (2006)
Khanafer, K., Vafai, K., Kangarlul, A.: Computational modeling of cerebral diffusion-application to stroke imaging. Magn. Reson. Imaging 21, 651–661 (2003)
Khashan, S.A., Furlani, E.P.: Effects of particle–fluid coupling on particle transport and capture in a magnetophoretic microsystem. Microfluid. Nanofluidics 12, 565–580 (2012)
Kim, D.H., Lipke, E.A., Kim, P., Cheong, R., Thompson, S., Delannoy, M., Suh, K., Tung, L., Levchenko, A.: Nanoscale cues regulate the structure and function of macroscopic cardiac tissue constructs. Appl. Biol. Sci. 107(2), 565–570 (2010)
Klinbun, W., Vafai, K., Rattanadecho, P.: Electromagnetic field effects on transport through porous media. Int. J. Heat Mass Transf. 55, 325–335 (2012)
Li, X., Yao, K., Liu, Z.: CFD study on the magnetic fluid delivering in the vessel in high-gradient magnetic field. J. Magn. Magn. Mater. 320, 1753–1758 (2008)
Loukopoulos, V.C., Tzirtzilakis, E.E.: Biomagnetic channel flow in spatially varying magnetic field. Int. J. Eng. Sci. 42, 571–590 (2004)
Lunnoo, T., Puangmali, T.: Capture efficiency of biocompatible magnetic nanoparticles in arterial flow: a computer simulation for magnetic drug targeting. Nanoscale Res. Lett. 10, 426 (2015)
Nacev, A., Beni, C., Bruno, O., Shapiro, B.: The behaviors of ferromagnetic nano-particles in and around blood vessels under applied magnetic fields. J. Magn. Magn. Mater. 323, 651–668 (2011)
Okuhata, Y.: Delivery of diagnostic agents for magnetic resonance imaging. Adv. Drug Deliv. Rev. 37, 121–137 (1999)
Pankhurst, Q.A., Connolly, J., Jones, S.K., Dobson, J.: Applications of magnetic nanoparticles in biomedicine. J. Phys. D: Appl. Phys. 36, 167–181 (2003)
Ramanujan, S., Pluen, A., McKee, T.D., Brown, E.B., Boucher, Y., Jain, R.K.: Diffusion and convection in collagen gels: implications for transport in the tumor interstitium. Biophys. J. 83, 1650–1660 (2002)
Saltzman, W.M.: Drug Delivery Engineering Principles for Drug Therapy. Oxford University Press, Oxford (2001)
Sarin, H.: Physiologic upper limits of pore size of different blood capillary types and another perspective on the dual pore theory of microvascular permeability. J. Angiogenes. Res. 2, 14 (2010)
Sharma, S., Singh, U., Katiyar, V.K.: Modeling and in vitro study on capture efficiency of magnetic nanoparticles transported in an implant assisted cylindrical tube under magnetic field. Microfluid. Nanofluidics 19, 1061–1070 (2015)
Shaw, S., Murthy, P.: Magnetic targeting in the impermeable microvessel with two-phase fluid model—Non-Newtonian characteristics of blood. Microvasc. Res. 80, 209–220 (2010)
Swartz, M.A., Fleury, M.E.: Insterstitial flow and its effects in soft tissues. Annu. Rev. Biomed. Eng. 9, 229–256 (2007)
Waite, L., Fine, J.: Applied Biofluid Mechanics. McGraw-Hill, Chicago (2007)
Wenger, M.P., Bozec, L., Horton, M.A., Mesquida, P.: Mechanical properties of collagen fibrils. Biophys. J. 93, 1255–1263 (2007)
Windberger, U., Bartholovitsch, A., Plasenzotti, R., Korak, K.J., Heinze, G.: Whole blood viscosity, plasma viscosity and erythrocyte aggregation in nine mammalian species reference values and comparison of data. Exp. Physiol. 88(3), 431–440 (2003)
Yuan, F., Dellian, M., Fukumura, D., Leunig, M., Berk, D.A., Torchilin, V.P., Jain, R.K.: Vascular permeability in a human tumor xenograft: molecular size dependence and cutoff size. Cancer Res. 55, 3752–3756 (1995)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ne’mati, S.M.A., Ghassemi, M. & Shahidian, A. Numerical Investigation of Drug Delivery to Cancerous Solid Tumors by Magnetic Nanoparticles Using External Magnet. Transp Porous Med 119, 461–480 (2017). https://doi.org/10.1007/s11242-017-0893-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11242-017-0893-1