Abstract
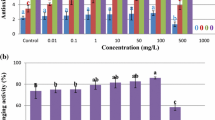
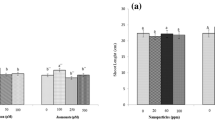
Copper oxide (CuO) nanoparticles (NPs) synthesized through co-precipitation method were employed in MS media during in vitro culture of Stevia rebaudiana. Physiological characteristics, production of steviol glycosides, and antioxidative parameters were investigated in regenerated plants. CuO NPs had crystalline monoclinic cubic cuprous oxides with average size 47 nm. The NPs were applied at 0, 0.1, 1.0, 10, 100 and 1000 mg/L in MS media for direct organogenesis of S. rebaudiana from nodal segments. Shoot organogenesis was found highest (88.5%) at 10 mg/L CuO and average shoot length, mean number of shoot per explant, and fresh weight were also found significantly higher at the same concentration. High performance liquid chromatography (HPLC) illustrated significant rise of bioactive major steviol glycosides (rebaudioside A and stevioside) at 10 mg/L CuO NPs in MS media. The oxidative stress produced by CuO nanoparticles on S. rebaudiana was affirmed by antioxidant activities i.e. total antioxidant activity (TAC), total reducing power (TRP) and 2,2-diphenyl-1-picryl hydrazyl (DPPH)-free radical scavenging activity. The oxidative stress generated by NPs involved production of antioxidative molecules total phenolic content (TPC), total flavonoid content (TFC) depending on NPs concentration. The study concludes that copper oxide nanoparticles functions as a stimulator of bioactive components productions, and can be employed in in vitro batch cultures.





Similar content being viewed by others
References
Ali A, Phull AR, Zia M, Shah AMA, Malik RN (2015) Phytotoxicity of River Chenab sediments: in vitro morphological and biochemical response of Brassica napus L. Environ Nanotechnol Monit Manag 4:74–84
Ali JS, Haq ul, Ali I, Ahmed A, Zia M, M (2017) Onosma bracteatum wall and Commiphora stocksiana Engl extracts generate oxidative stress in Brassica napus: an allelopathic perspective. Cogent Biol 3(1):1283875
Allam AI, Nassar AM, Besheit SY (2001) Itrogen fertilizer requirements of Stevia rebaudiana bertoni, under Egyptian conditions. Egypt J Agri Res 79:1005–1018
Allam A, El-Ghareeb AA, Abdul-Hamid M, Baikry A, Sabri MI (2011) Prenatal and perinatal acrylamide disrupts the development of cerebellum in rat: biochemical and morphological studies. Toxicol Ind Health 27(4):291–306
Aruoja V, Dubourguier HC, Kasemets K, Kahru A (2009) Toxicity of nanoparticles of CuO, ZnO and TiO 2 to microalgae Pseudokirchneriella subcapitata. Sci Total Environ 407(4):1461–1468
Aslani F, Bagheri S, Muhd Julkapli N, Juraimi AS, Hashemi FSG, Baghdadi A (2014) Effects of engineered nanomaterials on plants growth: an overview. Sci World J 2014. doi:10.1155/2014/641759
Boczkowski J, Hoet P (2010) What’s new in nanotoxicology? Implications for public health from a brief review of the 2008 literature. Nanotoxicol 4(1):1–14
Chang YN, Zhang M, Xia L, Zhang J, Xing G (2012) The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 5(12):2850–2871
Choi O, Hu Z (2008) Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environ Sci Technol 42(12):4583–4588
Cullity BD (1978) Elements of X-ray diffraction, Second edn. Addison-Wesley Publishing Company, Inc., Reading
Da Costa MVJ, Sharma PK (2016) Effect of copper oxide nanoparticles on growth, morphology, photosynthesis, and antioxidant response in Oryza sativa. Photosynthetica 54:110–119
Dahleen LS (1995) Improved plant regeneration from barley callus cultures by increased copper levels. Plant Cell Tiss Organ Cult 43(3):267–269
Dey A, Kundu S, Bandyopadhyay A, Bhattacharjee A (2013) Efficient micropropagation and chlorocholine chloride induced stevioside production of Stevia rebaudiana Bertoni. CR Biol 336:17–28
Dimkpa CO, Latta DE, McLean JE, Britt DW, Boyanov MI, Anderson AJ (2013) Fate of CuO and ZnO nano- and microparticles in the plant environment. Environ Sci Technol 47(9):4734–4742
Dimkpa CO, McLean JE, Latta DE, Manangón E, Britt DW, Johnson WP, Boyanov MI, Anderson AJ (2014) CuO and ZnO nanoparticles: phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J Nanopart Res 14:1–15
Dimkpa CO, McLean JE, Britt DW, Anderson AJ (2015) Nano-CuO and interaction with nano-ZnO or soil bacterium provide evidence for the interference of nanoparticles in metal nutrition of plants. Ecotoxicology 24(1):119–129
Ethiraj AS, Kang DJ (2012) Synthesis and characterization of CuO nanowires by a simple wet chemical method. Nanoscale Res Lett 7:1–5
Franklin NM, Rogers NJ, Apte SC, Batley GE, Gadd GE, Casey PS (2007) Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): the importance of particle solubility. Environ Sci Technol 41:8484–8490
Gajewska E, Skłodowska M (2007) Effect of nickel on ROS content and antioxidative enzyme activitiesin wheat leaves. Biometals 20:27–36
Garcia-Sago B, Roig LA, Moreno V (1991) Enhancement of morphogenetic response in cotyledon-derived explants of Cucumismelo induced by copper ions. Acta Hort 289:229–230
Haq IU, Mannan A, Ahmed I, Hussain I, Jamil M, Mirza B (2012) Antibacterial activity and brine shrimp toxicity of Artemisia dubia extract. Pak J Bot 44:1487–1490
Jain P, Kachhwaha S, Kothari SL (2009) Improved micropropagation protocol and enhancement in biomass and chlorophyll content in Stevia rebaudiana (Bert.) Bertoni by using high copper levels in the culture medium. Sci Hortic 119:315–319
Javed R, Usman M, Yücesan B, Zia M, Gürel E (2017a) Effect of zinc oxide (ZnO) nanoparticles on physiology and steviol glycosides production in micropropagated shoots of Stevia rebaudiana Bertoni. Plant Physiol Biochem 110:94–99
Javed R, Ahmed M, Haq ul, Nisa I, Zia S, M (2017b) PVP and PEG doped CuO nanoparticles are more biologically active: antibacterial, antioxidant, antidiabetic and cytotoxic perspective. Mat Sci Eng C 79:108–115
Jiang W, Mashayekhi H, Xing B (2009) Bacterial toxicity comparison between nano- and micro-scaled oxide particles. Environ Pollut 157:1619–1625
Kilam D, Saifi M, Abdin MZ, Agnihotri A, Varma A (2017) Endophytic root fungus Piriformospora indica affects transcription of steviol biosynthesis genes and enhances production of steviol glycosides in Stevia rebaudiana. Physiol Mol Plant Pathol 97:40–48
Klaper R, Crago J, Barr J, Arndt D, Setyowati K, Chen J (2009) Toxicity biomarker expression in daphnids exposed to manufactured nanoparticles: changes in toxicity with functionalization. Environ Pollut 157:1152–1156
Kołodziejczak-Radzimska A, Jesionowski T (2014) Zinc oxide—from synthesis to application: a review. Materials 7:2833
Kothari SL, Agarwal K, Kumar S (2004) Inorganic nutrient manipulation for highly improved in vitro plant regeneration in finger millets-Eleusine coracana (L.) Gaertn. In Vitro Cell Dev Biol-Plant 40:515–519
Kumar S, Narula A, Sharma M, Srivastava PS (2003) Effect of copper and zinc on growth, secondary metabolite content and micropropagation of Tinospora cordiofolia: a medicinal plant. Phytomorphol 53:79–91
Ladygin VG, Bondarev NI, Semenova GA, Smolov AA, Reshetnyav OV, Nosov AM (2008) Chloroplast ultrastructure, photosynthetic apparatus activities and production of steviol glycosides in Stevia rebaudiana in vivo and in vitro. Biol Plant 52:9–16
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Niedz RP, Evens TJ (2007) Regulating plant tissue growth by mineral nutrition. In Vitro Cell Dev Biol-Plant 43:370–381
Nirwan RS, Kothari SL (2003) High copper levels improve callus induction and plant regeneration in Sorghum bicolar (L.) Moench. In Vitro Cell Dev Biol-Plant 39:161–164
Pal PK, Prasad R, Pathania V (2013) Effect of decapitation and nutrient applications on shoot branching, yield, and accumulation of secondary metabolites in leaves of Stevia rebaudiana Bertoni. J Plant Physiol 170(17):1526–1535
Pande SD, Iqbal M, Srivastava PS (2000) Effect of ZnSO4 and CuSO4 on regeneration and lepidine content in Lepidium sativum L. Biol Plant 43:253–256
Pandey H, Pandey P, Pandey SS, Singh S, Banerjee S (2016) Meeting the challenge of stevioside production in the hairy roots of Stevia rebaudiana by probing the underlying process. Plant Cell Tiss Org Cult 126:511–521
Park SH, Kim HJ (2004) Unidirectionally aligned copper hydroxide crystalline nanorods from two-dimensional copper hydroxy nitrate. J Am Chem Soc 126:14368–14369
Perreault F, Samadani M, Dewez D (2014) Effect of soluble copper released from copper oxide nanoparticles solubilisation on growth and photosynthetic processes of Lemna gibba L. Nanotoxicol 8:374–382
Purnhauser L, Gyulai G (1993) Effect of copper on shoot and root regeneration in wheat, triticale, rape and tobacco tissue cultures. Plant Cell Tiss Org Cult 35(2):131–139
Regier N, Cosio C, von Moos N, Slaveykova VI (2015) Effects of copper-oxide nanoparticles, dissolved copper and ultraviolet radiation on copper bioaccumulation, photosynthesis and oxidative stress in the aquatic macrophyte Elodea nuttallii. Chemosphere 128:56–61
Rehman R, Chaudhary M, Khawar K, Lu G, Mannan A, Zia M (2014) In vitro propagation of Caralluma tuberculata and evaluation of antioxidant potential. Biologia 69(3):341–349
Sahrawat AK, Chand S (1999) Stimulatory effect of copper on plant regeneration in indica rice (Oryza sativa L.). J Plant Physiol 154(4):517–522
Shi J, Abid AD, Kennedy IM, Hristova KR, Silk WK (2011) To duckweeds (Landoltiapunctata), nanoparticulate copper oxide is more inhibitory than the soluble copper in the bulk solution. Environ Pollut 159:1277–1282
Song G, Hou W, Gao Y, Wang Y, Lin L, Zhang Z, Niu Q, Ma R, Mu L, Wang H (2016) Effects of CuO nanoparticles on Lemna minor. Bot Stud 57:1–8
Tahiliani S, Kothari SL (2004) Increased copper content of the medium improves plant regeneration from immature embryos derived callus of wheat (Triticum aestivum). J Plant Biochem Biotechnol 13:85–88
Utumi MM, Monnerat PH, Pereira PRG, Fontes PCR, Godinho VD (1999) Macronutri-ent deficiencies in Stevia: visual symptoms and effects on growth, chemical composition, and stevioside production. PesquiAgropecu Bras 34:1039–1043
Vives K, Andújar I, Lorenzo JC, Concepción O, Hernández M, Escalona M (2017) Comparison of different in vitro micropropagation methods of Stevia rebaudiana B. including temporary immersion bioreactor (BIT®). Plant Cell Tiss Organ Cult 131(1):195–199
Yadav V (2013) Nanotechnology, big things from a tiny world: a review. AEEE 3:771–778
Yu Q, Huang H, Chen R, Wang P, Yang H, Gao M, Peng X, Ye Z (2012) Synthesis of CuO nanowalnuts and nanoribbons from aqueous solution and their catalytic and electrochemical properties. Nanoscale 4:2613–2620
Yücesan B, Mohammed A, Büyükgöçmen R, Cevher A, Kavas Ö, Gürel S, Gürel E (2016) In-vitro and ex-vitro propagation of Stevia rebaudiana Bertoni with high Rebaudioside-A content—a commercial scale application. Sci Hortic 203:20–28
Zafar H, Ali A, Ali JS, Haq IU, Zia M (2016) Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: growth dynamics and antioxidative response. Front Plant Sci 7:535
Zafar H, Ali A, Zia M (2017) CuO nanoparticles inhibited root growth from Brassica nigra seedlings but induced root from stem and leaf explants. App Biochem Biotechnol 181:365–378
Zhao Y, Song X, Song Q, Yin Z (2012) A facile route to the synthesis copper oxide/reduced graphene oxide nanocomposites and electrochemical detection of catechol organic pollutant. Cryst Eng Commun 14:6710–6719
Zheng L, Liu X (2007) Solution-phase synthesis of CuO hierarchical nanosheets at near-neutral pH and near-room temperature. Mater Lett 61:2222–2226
Acknowledgements
Authors are grateful to The Scientific and Technological Research Council of Turkey, TUBİTAK (Program # 2216) for providing financial support. We are also thankful to the Department of Biology and Seed Science and Technology Abant Izzet Baysal University, Bolu, Turkey; Department of Biotechnology and Department of Physics, Quaid-i-Azam University, Islamabad, Pakistan for providing all the research facilities.
Author information
Authors and Affiliations
Contributions
RJ designed the study and wrote manuscript. AM and RJ performed experimental work. BY and RJ analyzed the data. MZ and EG critically reviewed the manuscript and added to its technical part. All authors have contributed, seen and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Silvia Moreno.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Javed, R., Mohamed, A., Yücesan, B. et al. CuO nanoparticles significantly influence in vitro culture, steviol glycosides, and antioxidant activities of Stevia rebaudiana Bertoni. Plant Cell Tiss Organ Cult 131, 611–620 (2017). https://doi.org/10.1007/s11240-017-1312-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-017-1312-6