Abstract
There are substantial variations in bulbing (bulb formation) efficiency among micropropagated tulip cultivars. The mechanisms involved are poorly understood, but presumably involve cytokinins (CKs) for several reasons. Therefore, we explored CK profiles and dynamics in ‘Blue Parrot’ and ‘Prominence’ cultivars (which have low and high bulbing efficiency, respectively) during the in vitro propagation stages: the last shoot multiplication subculture extended to 14 weeks (S1–S2), the shoot cooling at 5 °C for induction of bulb formation (S3–S4) and the bulb growth initiation after the end of cooling (S5–S6). The CK thidiazuron (TDZ) is routinely used in tulip micropropagation at the shoot multiplication stage, but replacing it with isopentenyladenine (iP) during the last multiplication subculture substantially changed CK dynamics in later stages, and significantly increased bulb formation rates in both cultivars. Generally, the most abundant CKs in both cultivars were the isoprenoid CK types, trans-zeatin (tZ), iP, cis-zeatin and dihydrozeatin. However, ‘Prominence’ shoots had much lower cis- to trans-Z-type CK ratios than ‘Blue Parrot’ shoots, and generally higher levels of physiologically active CKs (free bases tZ, iP and their ribosides) until the last phase of bulb formation, S6 (bulb growth initiation, i.e. swelling of shoot bases), 6 weeks after the end of cold treatment. In this phase total active CK and O-glucoside contents sharply declined in ‘Prominence’ shoots, but not in ‘Blue Parrot’ shoots pretreated with iP. In contrast, the low bulbing ability observed in ‘Prominence’ shoots pretreated with TDZ and ‘Blue Parrot’ shoots pretreated with either TDZ or iP was associated with a gradual rise in active CK and O-glucoside contents after the end of cooling. The results suggest that low bulbing efficiency may be related to down-regulation of tZ biosynthesis, and high bulbing efficiency to a transient increase in active CK forms (mainly tZs) in response to cold treatment during the bulb induction phase, S4 (at the end of cold treatment), followed by a rapid decrease during bulb formation, S6 (6 weeks after the end of cooling).
Similar content being viewed by others
Introduction
In vitro storage organ formation (tuberization) has been extensively studied in numerous geophytes (Ascough et al. 2008; Podwyszyńska 2012). Most geophytes require specific induction factors for storage organ formation, e.g. tulips require low temperatures, some onion genotypes require long photoperiods, and potatoes require short photoperiods and low night temperatures. However, numerous reports suggest that two factors induce an in vitro storage organ formation in most geophytes, including bulbous plants: (1) a high sucrose concentration in in vitro media and (2) a sharp reduction in endogenous gibberellin levels in response to environmental cues (Podwyszyńska 2012). The latter has been confirmed by demonstrations that application of gibberellin biosynthesis inhibitors stimulates bulb formation (inter alia) in garlic (Kim et al. 2003), lily (Kumar et al. 2005) and tulip (Podwyszyńska 2006b).
Several other plant growth regulators (PGRs) also have widely reported stimulatory effects on in vitro bulb formation (bulbing), including auxins (Van Aartrijk and Blom-Barnhoorn 1981), ethylene (Taeb and Alderson 1990) and jasmonates (Kim et al. 2003, Podwysyńska 2006a). However, there are conflicting indications of the roles of cytokinins (CKs) in bulb formation in vitro. Benzyladenine (BA) stimulated the bulb development in Lilium longiflorum (Nhut 1998) and Urginea maritima (El Grari and Backhaus 1987) but inhibited bulb formation in Narcissus jonquilla (Chow et al. 1992). A high cytokinin to auxin ration improved the bulb production in Hyacinthus (Kim et al. 1981) and Fritillaria fischeriana (Mirici et al. 2005). On the other hand, low exogenous cytokinin to auxin ratio reportedly promote bulb growth of Hippeastrum (Huang et al. 2005).
We recently published a novel protocol for in vitro tulip micropropagation (Podwyszyńska and Marasek 2003; Podwyszyńska and Sochacki 2010). This method involves cyclic multiplication of adventitious shoots on medium containing thidiazuron (TDZ) and 1-napthaleneacetic acid (NAA) followed by bulb formation (a key stage in tulip micropropagation as only bulbs have high rooting ability and good field performance) on hormone-free medium. It is generally based on previously reported methods for stimulating shoot organogenesis by applying benzyladenine (BA) and/or isopentenyladenine (iP) CKs in combination with NAA (Nishiuchi 1980; Rice et al. 1983; Le Nard et al. 1987). In these in vitro propagation protocols, tulip bulbs develop from shoots formed on initial explants isolated from floral stems or bulb scales. In our system, the bulbing process is directly induced in adventitious shoots multiplied in in vitro cycles over the course of 1–3 years.
We also discovered that modification and prolongation (to 14 weeks) of the last multiplication subculture prior to cooling, combined with applications of NAA and gibberellin biosynthesis inhibitors, enhance shoots’ bulbing capacity (Podwyszyńska 2006b). For these reasons, in our approach the bulb development stage takes 9–10 months and comprises three phases (Fig. 1). The in vitro bulb development process is similar to the process in natural conditions (Le Nard and De Hertogh 1993), and thus is controlled by complex sequences of interactions between internal and external factors. In the first phase, in which shoots are prepared for bulbing at a high ‘summer’ temperature of 20 °C, multiplication ceases, they develop dormancy, become sensitive to low temperature treatment, and their endogenous levels of abscisic acid (ABA) and indole-3-acetic acid (IAA), respectively rise and fall (Podwyszyńska et al. 2004). In the second phase bulb development is induced by exposing the shoots to a low ‘winter’ temperature treatment of 4–5 °C for 12–14 weeks. During this phase shoot dormancy is released, endogenous ABA levels fall while IAA levels rise. In the third phase, which usually takes 2–4 months, shoots are incubated on sucrose-rich medium at a high (‘spring’) temperature of 23 °C and form bulbs.
Tulip micropropagation and sampling schedule. CK measurements were done at successive phases of bulb formation stage: at eighth week of the last multiplication subculture (S1); 6 weeks after supplementation with the liquid medium containing NAA (at 14th week of the last multiplication subculture) (S2); at 12th week of cold treatment (S3); at 14th week (the end) of cold treatment, induction of bulb formation (S4); 2 weeks after cold treatment completion (S5); 6 weeks after cold treatment completion (at the shoot base swelling, initiation of bulb formation) (S6)
Under this sequence of treatments, more than 80 % of the shoots of most tulip genotypes, including our model cultivar ’Prominence’, form bulbs. However, some genotypes have low bulbing capacity under these conditions, notably cultivars of Parrot group such as ‘Blue Parrot’ and ‘Black Parrot’ (Podwyszyńska 2001, 2006b), which arose as mutants of non-Parrot (mainly Single Late) cultivars (Van Scheepen 1996). In these tulips, bulb formation frequency is lower than 40 %, bulbs take as long as 5 months to develop, and many bulbs are irregular. Furthermore, the low bulbing capacity of these cultivars is associated with high shoot multiplication ability, and in most Parrot tulips both flowering and bulb maturation occur late in the season (Van Scheepen 1996). In vivo some Parrot cultivars also generally produce relatively high numbers of daughter bulbs, but they are often small and thus have low quality (Sochacki D., personal communication).
Saniewski and Mynett (1977) suggested that Parrot cultivars may have constitutively high levels of endogenous CKs since they found that parrot-like flowers developed from BA-treated bulbs of non-Parrot cultivars. The hypothesis that endogenous CK status could influence the tuberization process is supported by findings that exogenous iP induces bulb formation far more effectively than exogenous BA (Le Nard et al. 1987). The cited authors suggested that since BA is more stable than iP it could accumulate in shoots and subsequently inhibit bulb development. In our micropropagation method we apply TDZ, as we found it was essential for cyclic multiplication of adventitious shoots in tulip (Podwyszyńska and Marasek 2003). This phenylurea compound with strong cytokinin-like activity is very stable (Mok and Mok 2001) and has been used in plant tissue culture for over 40 years because it has high morphogenetic potential (Huetterman and Preece 1993; Guo et al. 2011). It induces many responses typical of naturally occurring CKs (Mok and Mok 2001), including strong inhibition of leaf senescence (Ferrante et al. 2003; Mortazavi et al. 2011). Since bulbing in tulip is always connected to yellowing and withering of leaves, from which nutrients are transported to developing bulbs, application of TDZ in vitro could perturb the process by retarding leaf senescence.
Since CKs clearly play important, but poorly understood, roles in bulb formation, in the presented study we tested the hypothesis that variations in tulip genotypes’ ability to form bulbs are related to CK dynamics and profiles. In addition, we tested the hypothesis that replacing TDZ with iP in the last multiplication subculture period (prior to cooling) could enhance subsequent bulb formation, if so the mechanisms involved, and effects of these exogenous PGRs on endogenous CK status during the following bulb development phases. The results also provide the first detailed description of the endogenous CK composition of micropropagated tulips.
Materials and methods
Plant material and in vitro culture conditions
Two model tulip cultivars differing substantially in bulb formation capacity were used in the study: ‘Blue Parrot’ and ‘Prominence’, which form bulbs poorly and readily in vitro, respectively (Podwyszyńska 2006b). ‘Blue Parrot’ (a mutant of the Single Late cultivar ‘Bleu Aimable’) is a late-flowering Parrot tulip that generally produces relatively small bulbs, while ‘Prominence’ is a cultivar of the Triumph group that produces a single flower in mid-season. As outlined in Fig. 1, in vitro shoot cultures of both cultivars were established, multiplied by adventitious regeneration, and prepared for bulb formation essentially following our previously published protocol (Podwyszyńska and Marasek 2003; Podwyszyńska and Sochacki 2010). Briefly, the shoots were continuously multiplied in vitro with 8-week subculture periods in 330 ml jars containing 40 ml of multiplication medium (Podwyszyńska and Sochacki 2010) supplemented with 1 µM TDZ and 1 µM NAA solidified with 6 g l−1 agar (Difco), until the last multiplication subculture, when TDZ was used at lower concentration of 0.5 µM TDZ or replaced with 25 µM iP. Effects of these exogenous PGRs on both morphological changes and endogenous CK contents were then monitored during the following phases of in vitro bulb development in both tulip cultivars. After 8 weeks a liquid medium (35 ml per jar) containing Murashige and Skoog (1962) medium (MS) and 5 µM NAA was added, and the shoots were grown on the resulting two-phase medium for a further 6 weeks (thus the last subculture before cooling was prolonged to 14 weeks). Then, the shoots were transferred in undivided clusters to MS medium (60 ml per jar) containing 7 % sucrose and 0.1 % activated charcoal but no PGRs, and the jars were kept at 5 °C for 14 weeks to induce bulbing process. Five jars, each containing five clumps of 3–5 shoots (20 shoots per jar) were used for each treatment in the in vitro bulb formation efficiency experiment. Finally, to initiate bulb formation the shoots were cultured (on the same medium) for 14 weeks at 23 °C with 16/8 h photoperiods (illuminated at a photosynthetic photon flux density of 55 µmol m−2 s−1 by warm-white fluorescent lamps during the light phases).
The total number of bulbs and the number of regular bulbs (properly formed and covered with tunic) obtained from each jar as well as mean bulb weight were recorded at the end of the experiment, 14 weeks after the end of cooling. The efficiency of bulb formation was expressed as the ratio of the number of regular bulbs to shoot number (n = 20) × 100. There were five replications (data from each jar) per treatment. The bulb formation data were subjected to a two factor analysis of variance in relation to genotype and exogenous TDZ/iP pretreatment (ANOVA) (STATISTICA package StatSoft v. 10). The means were compared by Duncan’s test at P = 0.05.
Determination of cytokinins
The endogenous CK profiles of the shoots were monitored by the following sampling, extraction and high-performance liquid chromatography-mass spectrometry (LC–MS) procedures. Additional four jars per treatment containing tulip shoots cultured as described above, were maintained for sampling. Samples of basal parts of shoots (ca. 1 cm long and 1 g fresh weight) were taken at the following stages of micropropagation/bulb development (Table 1; 28 weeks in total): S1, at the eighth week of the last multiplication subculture; S2, at the 14th week of the last multiplication subculture, just before the cold treatment (6 weeks after addition of the liquid medium containing NAA); S3, at 12th week of cold treatment; S4, after 14 weeks (the end) of cold treatment; S5, 2 weeks after the end of cold treatment: S6, 6 weeks after the end of cold treatment (during the shoot base swelling, i.e. initiation of bulb formation). The samples were frozen at −20 °C, lyophilized and stored at −20 °C until analyses.
Triplicate samples containing plant material collected from at least two jars representing each treatment were extracted for 3 h in ice-cold 70 % ethanol (v/v), then re-extracted for 1 h in fresh solvent. A mixture of 45 deuterium-labelled cytokinin standards (1 pmol per standard) was added to each sample to validate determinations using the isotope dilution method. After extraction, the combined supernatants were concentrated to approximately 1.0 ml under vacuum at 35 °C, diluted to 20 ml with ammonium acetate buffer (40 mM, pH 6.5) and purified using tandem DEAE-Sephadex (1.0 × 5.0 cm)-octadecylsilica (0.5 × 1.5 cm) columns and an immunoaffinity chromatography (IAC) column containing a bound generic CK monoclonal antibody (Faiss et al. 1997). This resulted in three fractions containing: (1) the free bases, ribosides and N-glucosides; (2) a ribotide fraction; and (3) an O-glucoside fraction. Fractions 2 and 3 were also purified by IAC, after treatment with alkaline phosphatase (Roche Diagnostics, Mannheim, Germany) and β-glucosidase (G-0395, Sigma, St. Louis, USA), respectively. Eluates from the IAC columns were evaporated to dryness and dissolved in 50 µl of the mobile phase used for quantitative analysis (see below).
The LC–MS analysis was carried out (following Novák et al. 2003) using an HPLC Alliance 2695 Separations Module (Waters, Milford, MA) linked to a photo diode array (PDA 996, Waters) and ZMD 2000 single quadrupole mass spectrometer equipped with an electrospray interface [LC(+)ESI–MS] (Micromass, Manchester, UK). The samples (25 µl) were injected onto a C18 reversed-phase column (Waters; Symmetry; 5 µm; 150 mm · 2.1 mm) and eluted with a 25 min linear gradient from 10:90 to 50:50 ratios of A (100 % methanol) to B (15 mM HCOONH4, pH 4), followed by a 5 min isocratic wash with 50:50 A:B. Using a 1:1 post-column split, the effluent was simultaneously introduced into the diode array detector and the electrospray source (source block temperature 100 °C, desolvation temperature 250 °C, capillary voltage +3.0 V, cone voltage 20 V). CKs were quantified by selected-ion-monitoring of their precursor ions ([M + 1]+) and the corresponding labelled internal standards.
Means of CK contents and standard deviations (SD) (n = 3) were calculated. Data were subjected to a three-factor analysis of variance ANOVA in relation to cultivar, TDZ/iP pretreatment, and bulbing phase. Due to the violation of the homogeneity of variance assumption verified by the Levene’s Test data were transformed previously using Box-Cox procedures. All calculations were done with the STATISTICA package (StatSoft v. 10).
Results
Efficiency of bulb formation
Under in vitro conditions each tulip shoot has an apical meristem enclosed by the base of a single rolled leaf. After completion of the cooling, the leaves grow intensively. The first morphological symptoms of bulb formation are cessation of leaf growth, swelling of shoot bases and their progressive yellowing. As shown in Fig. 2a, under our culture conditions these symptoms appeared in ‘Prominence’ shoots within 6 weeks after completion of the cooling. In contrast, ‘Blue Parrot’ shoots remained green, their leaves grew and no bulbing symptoms appeared for 9–10 weeks following the cooling period. Furthermore, the bulbing symptoms were weaker and less synchronic than in ‘Prominence’. Some ‘Blue Parrot’ shoots even remained green and did not swell until the end of the cultivation period, thus their bulb formation was generally delayed and inefficient (Fig. 2b). After completion of the cooling, shoots of ‘Prominence’ developed bulbs within 9 weeks while the first ‘Blue Parrot’ bulbs formed 5 weeks later. TDZ and iP applied in the last multiplication subculture had markedly contrasting effects on subsequent bulb formation. Using iP instead of TDZ in the multiplication medium significantly increased the percentage of ‘Prominence’ and ‘Blue Parrot’ shoots that formed regular, suitable bulbs for cultivation from 62.7 to 96.1 % and from 20.0 to 39.3 %, respectively (Table 1; Fig. 2b).
In vitro bulb formation of tulip: on both photographs ‘Prominence’ on the left and ‘Blue Parrot’ on the right. Shoots forming bulbs, 8 weeks after cooling completion (a). Bulbs obtained from shoot cultures derived from the medium containing TDZ (top) or iP (bottom) (b); regular bulbs (left) and irregular (right)
Endogenous cytokinins contents
General CK patterns and dynamics
In the LC–MS analyses of 45 CKs potentially present during the monitored bulb development phases, 19 were identified and quantified (Table 3). No benzyladenine-riboside (BAR), benzyladenine-9-glucoside (BA9G), benzyladenosine-5′-monophosphate (BARMP), kinetin-riboside (KR), kinetin-riboside-5′-monophosphate (KRMP) or any of 17 topolin derivatives (meta-, ortho-, and para-topolin bases, ribosides, nucleotides and glucosides) were detected. The presence of BA (≤2.92 pmol g−1 FW) and kinetin (≤0.09 pmol g−1 FW) is unusual, and could have originated from contamination (data not presented). Generally, the most abundant CKs in both cultivars were the isoprenoid CK types, trans-zeatin (tZ), iP, cis-zeatin (cZ) and dihydrozeatin (DZ) as well as their derivatives, ribosides, nucleotides (NTs) and O-glucosides (OGs). However, distinct differences in endogenous CK levels and patterns were observed between cultivars, TDZ and iP pretreatments, and shoot/bulb developmental stages (Tables 2, 3; Fig. 3). Probability value (P) of F test from three-factor analysis of variance of the endogenous CKs contents in shoots of tulip two cultivars during the six phases of bulb formation, in relation to the exogenous TDZ/iP pretreatments are presented in Table 2. These P values indicate that the most significant factor influencing changes in CK contents was the phase of shoot development. Contents of all of the endogenous CKs significantly changed in the successive phases. Contents of thirteen CKs were significantly influenced by the exogenous TDZ/iP pretreatments and contents of twelve CKs were significantly dependent on the cultivar.
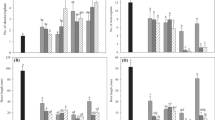
Total contents (pmol g−1 FW) of free CK bases (a), ribosides (b), NTs (c) and OGs (d) in the in vitro shoots of the tulip cultivars ‘Prominence’ and ‘Blue Parrot’ during bulb formation process following pretreatment with TDZ or iP. CK measurements were done at successive phases S1, S2, S3, S4, S5 and S6 as described in sampling schedule (Fig. 1). The total content of bases consists of iP, tZ, cZ and DZ; ribosides: iPR, tZR, cZR and DZR; NTs, nucleotides: iPRMP, tZRMP, cZRMP and DZRMP; OGs, O-glucosides: tZOG, tZROG, cZOG, cZROG, DZOG and DZROG. Data represents mean ± SD of three replication per treatment
Prominence’ shoots generally had higher levels of active CKs, free bases and ribosides of iP, tZ and DZ as well as their nucleotides, than ‘Blue Parrot’ shoots, throughout the micropropagation process except in the last phase of bulb formation, 6 weeks after completion of the cold treatment (S6) (Table 3, Fig. 3a–c). In this phase, iP-pretreated ‘Prominence’ shoots had total CK ribosides’ content (18.03 pmol g−1 FW) much lower compared to ‘Blue Parrot’ (98.28 pmol g−1 FW). Whereas the contents of total CK free bases were similar, ca. 35 pmol g−1 FW, in both cultivars at this phase.
In comparison to TDZ-pretreatment, the iP-pretreatment resulted at S1 in ca. 63- and 24-fold higher total endogenous CK levels (predominantly iP) in ‘Prominence’ (1,130.5 pmol g−1 FW) and ‘Blue Parrot’ (339.05 pmol g−1 FW) shoots, respectively (Table 3). In the following 6 weeks to S2, just before cooling, the total CK contents declined by ca. 80 and 75 % in ‘Prominence’ and ‘Blue Parrot’, respectively. In TDZ-pretreated shoots, similar reductions in CK levels (by ca. 84 and 50 %, respectively) were observed in ‘Prominence’, but not ‘Blue Parrot’, shoots between S1 and S2. CKs contents subsequently rose again to ca. 540–460 pmol g−1 FW in ‘Prominence’ (S4–S5) and 170 pmol g−1 FW in ‘Blue Parrot’ (S3), respectively, mainly due to the accumulation of iPs and tZs. The CK levels in the iP-pretreated ‘Prominence’ shoots then declined and were ca. 50 % lower 6 weeks after completion of the cooling (S6). In contrast, levels of total CKs significantly increased in both iP and TDZ-pretreated ‘Blue Parrot’ shoots at this stage by ca. 67 and 165 %, respectively.
CK bases
These active cytokinin forms include iP, tZ, cZ and DZ. The substitution of TDZ by iP in the multiplication medium had strongest effects on ‘Prominence’ shoots, resulting in extremely high iP base contents at stage S1, at eighth week of the last multiplication subculture (1,107.30 pmol g−1 FW), threefold higher than in ‘Blue Parrot’ counterparts (Fig. 3a; Tables 2, 3). The levels slowly declined to 27.57 (‘Prominence’) and 31.77 (‘Blue Parrot’) pmol g−1 FW 6 weeks after the end of cooling period (S6). Other CK bases occurred at lower concentrations. Total CK base contents also followed this trend, except S4–S5 (at the end of cooling period and 2 weeks later) when they increased slightly to ca. 118 pmol g−1 FW in ‘Prominence’ shoots than decreased again at to ca. 33 pmol g−1 FW (S6). In TDZ-pretreated shoots of both cultivars, total CK base content was drastically lower (0.81–10.53 pmol g−1 FW) with the highest content at S6 in ‘Prominence’, mainly due tZ and cZ.
CK ribosides
This group of active CKs comprises isopentenyladenosine riboside (iPR), trans-zeatin riboside (tZR), cis-zeatin riboside (cZR) and dihydrozeatin riboside (DZR). Changes in their levels substantially differed from those of CK free bases (Fig. 3b). At S1 and S2, total riboside contents were low (1.19–4.98 pmol g−1 FW) in both cultivars and TDZ or iP pretreatments. During the cooling (S3–S4) and 2 weeks after the end of cooling (S5), their contents slightly increased in the TDZ or iP pretreated ‘Blue Parrot’ shoots and picked to ca. 61 and 98 pmol g−1 FW at S6 for TDZ and iP pretreatments, respectively. TDZ-pretreated ‘Prominence’ shoots followed this trend but rapid growth of ribosides occurs earlier at S5 reaching at S6 113.36 pmol g−1 FW. In contrast, iP-pretreated ‘Prominence’ shoots contained the highest amount of ribosides (51.62 pmol g−1 FW) at the end of cooling period (S4). Then their contents decreased to 18.03 pmol g−1 FW. The most abundant riboside was tZR, followed by iPR and cZR (Table 3).
CK nucleotides
The group of nucleotides, CK biosynthetic products, covers dihydrozeatin riboside-5′-monophosphate (iPRMP), trans-zeatin riboside-5′-monophosphate (tZRMP), cis-zeatin riboside-5′-monophosphate (cZRMP) and dihydrozeatin riboside-5′-monophosphate (DZRMP). The trends in NT dynamics were generally similar to those of ribosides. At S1, total nucleotide concentrations were below 9 pmol g−1 FW under both pretreatments (Fig. 3c). They then gradually increased to ca. 183 pmol g−1 FW in TDZ-pretreated ‘Blue Parrot’ shoots at S6 and, more strongly (to ca. 267 pmol g−1 FW) in TDZ-pretreated ‘Prominence’ shoots at S5. In iP-pretreated ‘Prominence’ shoots, nucleotide peaks were observed even earlier (S4, the end of the cooling period), then NT content decreased significantly to ca. 174 pmol g−1 FW at shoot swelling (S6). In this cultivar, NT content lowered also at this stage in TDZ pretreated shoots. The iP pretreatment more strongly induced biosynthesis of NT, mainly iPRMP, especially in the cytokinin-sensitive cultivar ‘Prominence’ (235.05 pmol g−1 FW of iPRMP at S4 and 172.66 pmol g−1 FW of tZRMP at S6) (Table 3). The accumulation of NT was usually followed by increases in free base, riboside and O-glucoside levels, most strongly in ‘Prominence’.
O-glucosides
These storage CKs are represented by trans-zeatin-O-glucoside (tZOG), cis-zeatin-O-glucoside (cZOG), trans-zeatin riboside-O-glucoside (tZROG) cis-zeatin riboside-O-glucoside (cZROG), dihydrozeatin-O-glucoside (DZOG) and dihydrozeatin riboside-O-glucoside (DZROG). Contents of OGs, were generally higher in ‘Blue Parrot’ than in ‘Prominence’ throughout the bulb development process (Fig. 3d). In ‘Blue Parrot’, the highest OG levels were recorded 6 weeks after the end of cooling (S6, 31.92 and 50.02 pmol g−1 FW following TDZ- and iP-pretreatment, respectively). In ‘Prominence’, total O-glucoside levels were significantly lower in this phase (24.21 and 4.72 pmol g−1 FW, following TDZ- and iP-pretreatment, respectively). The most abundant OG species were cZROG in ‘Blue Parrot’ and tZROG in ‘Prominence’ (Table 3).
Dihydrozeatin derivatives
This CK group includes DZ, dihydrozeatin riboside (DZR), dihydrozeatin riboside-5′-monophosphate (DZRMP), dihydrozeatin-O-glucoside (DZOG) and dihydrozeatin riboside-O-glucoside (DZROG). These derivatives were detected in micropropagated tulips generally at very low levels (Table 3). Before cooling, their concentrations were lower than 0.5 pmol g−1 FW. Following TDZ-pretreatment total DZ levels subsequently rose to peaks of 15.28 and 5.88 pmol g−1 FW at S6 in ‘Prominence’ and ‘Blue Parrot’, respectively. In contrast, levels of DZ derivatives were highest at the end of the cooling period (S4) in the iP-pretreated shoots, and higher in ‘Prominence’ than in ‘Blue Parrot’ shoots, especially DZROG (12.20 and 5.52 pmol g−1 FW, respectively) and DZRMP (17.05 and 4.17 pmol g−1 FW, respectively). The temporal patterns of changes in levels of DZ metabolites were similar to those observed for tZ CKs.
9-Glucosides
The only inactive CK form detected in shoots of both cultivars was trans-zeatin-9-glucoside (tZ9G). It was mostly present (at low concentrations, below 2.20 pmol g−1 FW) at the end of the cooling period (S4) and 2 weeks after the end of cooling (S5) ((Table 3). Levels were particularly low in ‘Blue Parrot’ (Table 3). The only significant rise in levels, to 4.54 pmol g−1FW, was recorded at the end of cooling (S4) in TDZ-pretreated ‘Prominence’ shoots. Interestingly, iP-treatment led to reductions in 9-glucoside levels in both cultivars.
Zeatin cis to trans isomer ratio
Among zeatin isomers, in ‘Prominence’ shoots tZ-type CKs were generally most abundant except during S1, at eighth week of the last multiplication subculture, when there was a striking dominance of cis isomers (cis/trans ratio: 39.16) in TDZ-pretreated shoots (Table 4). In contrast, the more abundant zeatin compounds in ‘Blue Parrot’ were cis-isomers. However, in both cultivars the ratio of cis- to trans-isomers was highest at the multiplication stage (S1), although higher in ‘Blue Parrot’ (cis/trans ratio: 72.37 and 50.61 at stages S1 and S2, respectively) than in ‘Prominence’ shoots. Furthermore, cis/trans-isomer ratios were much higher in ‘Blue Parrot’ than in ‘Prominence’ shoots in all bulb development phases (Table 4). In ‘Blue Parrot’ these ratios were generally higher than 1:1 except in the last phase S6, following both TDZ- and iP-pretreatments, and at S4 following iP-pretreatment. In addition, TDZ pre-treatment resulted in consistently higher cis/trans isomer ratios than iP pre-treatment. However, there were a few exceptions to these patterns. For example, cZR concentrations were higher in ‘Prominence’, and the changes in its concentrations were much stronger, than in ‘Blue Parrot’ shoots (Table 3).
Discussion
The action, metabolism and translocation of CKs in plants have been comprehensively reviewed (Sakakibara 2006; Hirose et al. 2008; Werner and Schmülling 2009). These PGRs play crucial roles in regulating diverse plant development and physiological processes, including cell proliferation and differentiation, seed germination, apical dominance, flower and fruit development, leaf senescence, and transduction of nutritional signals. However, little is known about their roles in storage organ formation (Sarkar et al. 2006; Malkawi et al. 2007). Naturally occurring CKs are adenine derivatives carrying an isoprene-derived or aromatic side chain at the N6-amino group. Numerous reports indicate that isoprenoid types are more abundant than aromatic types in diverse plants, and the most abundant isoprenoid types include tZ, iP, cZ and DZ (Hirose et al. 2008).
Our results show that micropropagated tulips share these general CK patterns, but their CK profiles are strongly influenced by the exogenous PGRs (TDZ and iP) used at the stage of shoot multiplication, the tulip genotype, and the developmental phase. Similar large differences in cytokinin patterns depending on exogenously applied CKs and plant growth stages were found in micropropagated Merwilla plumbea (monocot bulbous plant) (Aremu et al. 2014). As in our study, the isoprenoid CKs (mainly iPs) were the most abundant, especially in plant material treated with iP. Moreover, among endogenous isoprenoid CKs, the levels of DHZ were the lowest both in our tulip genotypes and M. plumbea. iP-treatment was also associated with lower accumulation of 9-glucosides as compared to treatments with aromatic CK (BA) (Aremu et al. 2014) or TDZ (our study). Furthermore, exogenous iP (in comparison with aromatic CKs) applied during in vitro shoot multiplication influenced more favorably rooting and growth of M. plumbea during acclimatization, the final micropropagation stage. This corresponds with our results. Bulbing in the tulip shoot cultures was influenced more efficiently by iP than by TDZ, confirming the hypothesis that replacing TDZ with iP in the last multiplication subculture period (prior to cooling) could enhance subsequent bulb formation. This is consistent with the finding by Le Nard et al. (1987) that iP-containing medium induces bulb formation far more effectively than BA-containing medium. It should be emphasized that in both our studies and those of Le Nard et al. (1987) no exogenous CKs were used in the medium during late phases of the bulbing process, i.e. their induction by cooling shoots and bulb growth. Thus, the findings clearly indicate that TDZ and BA have prolonged effects on the in vitro bulbing of tulips.
TDZ-pretreatment probably inhibits bulb formation (relative to iP-pretreatment) by reducing changes in CK profiles during bulb induction and initiation. The differences in CK dynamics between TDZ and iP pretreatments were more pronounced in ‘Prominence’, in which (unlike iP) TDZ induced neither a clear peak of CK accumulation after cooling nor a subsequent decline in CK contents, both of which are probably required for efficient bulb formation. Instead, following TDZ-pretreatment contents of active CKs (free bases and ribosides) and O-glucosides tended to increase throughout the experiment while contents of CK nucleotides started to decrease at most 6 weeks after completion of the cold treatment (S6). This implies that despite its absence in the medium for more than 20 weeks TDZ continued to affect CK metabolism in late developmental phases. Thus, this very stable phenylurea may remain in tissues and continue to perturb CK profiles and signaling for a long time (months), as recorded in Phaseolus lunatus callus tissue treated with TDZ (Capelle et al. 1983). TDZ reportedly acts by directly binding to CK receptors (Spíchal et al. 2004) or indirectly by inhibiting CK oxidase/dehydrogenase (CKX) activity (Hare and van Staden 1994; Zatloukal et al. 2008). Both of these activities could contribute to the effects observed in our study. There are several differently targeted isoforms of this enzyme (CKX, EC 1.5.99.12) in plant cells, which can greatly differ also in their preference for cis- or trans-zeatin as a substrate (Šmehilová et al. 2009). However, because relative inhibitory activity of TDZ against different isoforms of CKX is not known, TDZ treatment can not only influence endogenous cytokinin content (in comparison with iP pretreatment), but also cis/trans-zeatin ratio in targeted tissue, which can lead to changes in bulb formation capacity.
The most pronounced difference between the tulip cultivars we investigated was that ‘Blue Parrot’ had much higher ratios of cis- to trans-Z-type CKs than ‘Prominence’, which has substantially higher bulbing capacity. In ‘Prominence’ shoots, cZs prevailed over tZs only at the end of the multiplication phase (before cooling, stage S2), which is associated with dormancy development (Podwyszyńska et al. 2004). In contrast, in ‘Blue Parrot’ shoots, cis-isomers were the predominant Z-type CKs in all of the phases except the last, bulb growth initiation (S6, 6 weeks after completion of the cooling), when tZ-type CKs dominated. At that time, bulb growth should be initiated simultaneously with dormancy development, manifested by leaf yellowing. These phenomena were highly visible in ‘Prominence’ shoots pretreated with iP, but in ‘Blue Parrot’ shoots the shoot bases swelled sporadically and leaves remained green following both iP- and TDZ-pretreatment. However, in both cultivars and following both exogenous CK pretreatments, the absolute cZ-type CK contents progressively rose during the experiment and were highest 2 or 6 weeks after completion of the cooling (S5 and S6), except at the end of the cooling period (S4), when they slightly declined. These results are consistent with findings presented by Gajdošová et al. (2011), who postulated that cZ-type CKs are subtle regulators of CK responses in plants under growth-limiting conditions. They found that levels of cis- and trans-Z differ during the ontogenesis of Arabidopsis, ratios of cis- to trans-CKs increased in mature leaves and seeds (at an active growth cessation). A similar CK profile has also been detected in potato plants during the tuberization process, which is associated with dormancy development (Malkawi et al. 2007), as in tulip bulb formation (Podwyszyńska et al. 2004). Our results correspond also with observed increases in cZR levels in shoot apices of Brassica napus plants during prolonged exposure to low temperature, when the shoot meristems switch from vegetative to reproductive morphogenetic programs and form flowers instead of leaves (Tarkowská et al. 2012). Similarly in tulip in vitro cultures, low temperatures induce a switch to the development of storage organs, and shoot apical meristems form scales instead of leaves. Furthermore, under natural conditions, low temperatures during the winter induce both flower and daughter bulb development (Le Nard and De Hertogh 1993), and ZR levels may apparently be upregulated by specific inductive factors (e.g. low temperature for bulb/flower development in tulip and flower induction in oilseed rape (Tarkowská et al. 2012), or short photoperiods for tuberization in potato, according to Malkawi et al. (2007).
Our results confirmed the findings of Gajdošová et al. (2011) that cZs are more prevalent than previously believed. These CKs were detected in more than 150 plant species including Liliales, the order covering tulips. Recently, high quantities of cZs were detected in micropropagated monocot bulbous plant Mervilla plumbea (Aremu et al. 2014).
cZ has long been considered inactive or less active than tZ (Schmitz et al. 1972; Kamínek et al. 1987). Gajdošová et al. (2011) confirmed that cZs had weaker biological activity in several bioassays than their trans-counterparts. However, there was an exception: cZR was found to be only slightly less effective than tZR. Bassil et al. (1993) suggested that cis-isomers might function as precursors or transport forms of Z. The latter function was corroborated by Hiroshe et al. (Hirose et al. 2008) in Arabidopsis. tZR was identified as a major CK in xylem sap, it was assigned a role as an acropetal signal. In contrast, phloem sap contained predominantly iPR and cZR, which were postulated to function as basipetal or systemic signals. Hiroshe et al. (Hirose et al. 2008) further suggested that the two pathways might co-operatively regulate plant growth and development responses to environmental changes. Late rises in tZ and tZR contents of tulip shoots we observed at S6 (the bulbing initiation) were associated with inefficient, retarded bulb formation and the absence of leaf senescence symptoms. Thus, accumulation of these CK metabolites in shoots may disturb bulb formation by interfering with CK transport and thus basipetal transport of assimilates from the leaves to developing bulb scales, i.e. transformed leaves in growing bulbs.
Our endogenous CK measurements are closely correlated with observed morphological changes during bulbing. The first morphological symptoms of bulb formation are leaf base swelling accompanied by cessation of leaves’ growth and yellowing. In the iP-pretreated ‘Prominence’ shoots such symptoms occurred within 6 weeks after completion of their cooling (S6) and were closely associated with reductions in endogenous CK contents. In contrast, ‘Blue Parrot’ shoots remained green, their leaves continued to grow and changes in their endogenous CK levels were weaker and retarded. Furthermore, with TDZ-pretreatment these trends were observed in both cultivars, and they had high ratios of cis- to trans-Z type CKs during early stages (S1 and S2 in ‘Prominence’ and S1–S5 in ‘Blue Parrot’), due to very low levels of tZ-types. These findings indicate that TDZ may specifically stimulate the biosynthesis of cZ-type CKs, which putatively involves a different pathway from tZ type synthesis (Kasahara et al. 2004). In accordance with this hypothesis, pretreatment by iP not only stimulated the accumulation of endogenous iP CK metabolites, but also clearly induced increases in levels of all tZ CKs in both cultivars, most strongly in ‘Prominence’. In contrast, it had very little (if any) stimulatory effect on levels of cZs and DZs, as a strong decrease in these CKs in the last two phases was observed in iP-pretreated ‘Prominence’ shoots. These our results corresponds well with the report of Ǻstot et al. (2000) who discovered two biosynthetic pathways for zeatin-type CKs: iPRMP-dependent and iPRMP-independent pathway. Later it was discovered that in higher plants, the dominant pathway of tZ synthesis, is trans-hydroxylation of iP-type CKs, catalysed by a P450 monooxygenase (Kasahara et al. 2004; Hwang and Sakakibara 2006). On the other hand, a large fraction of cZ side chain is derived from the mevalonate, iPRMP-independent pathway (Kasahara et al. 2004; Hwang and Sakakibara 2006). This is in good agreement with our results, where iP pretreatment in the last multiplication subculture period (S1–S2, prior to cooling) greatly lowered cis/trans zeatin ratio in comparison with TDZ treatment, and subsequently enhanced bulb formation, which was shown by us to be strongly dependent on this ratio. The high endogenous iP levels probably stimulate its conversion to tZs, as described by Takei et al. (2004). iP could also enhance the activity of zeatin cis–trans-isomerase, as reportedly detected in immature Phaseolus seeds (Bassil et al. 1993). Based on our findings, we recommend in tulip micropropagation to replace TDZ with iP in the last multiplication subculture. Such iP pretreatment markedly improves bulb formation efficiency in the number of tulip genotypes, including Parrot cultivars propagated in vitro with our method (Podwyszyńska and Sochacki 2010).
We conclude that high bulbing ability in tulips may be associated with a transient increase in active CK forms in response to cold treatment during the bulb induction phase (at the end the cooling, S4), and a subsequent decline in their concentrations during the bulb growth initiation, when shoot bases begin to swell (S6). During induction (S4) the free bases and ribosides of tZ and iP were the most abundant active CKs. These findings are generally consistent with reported rises in active CK contents (especially cZR) in potato plants under tuber-inducing conditions, after 4 days exposure to short photoperiods (Malkawi et al. 2007). The increase in cZR contents observed in the cited study was followed by a rapid decline just before stolon swelling, the first tuber formation symptom (after 8 days under inductive conditions). Thus, there are clear similarities between tulip bulb and potato tuber formation processes.
Due to the fact that no detailed description of the endogenous CK composition has been found for bulbous plants we discuss several aspects of tulip bulb formation using examples concerning the tuber formation of potato as a model plant, in which the process has been studied most widely. It seems that the mechanisms of storage organ formation (on biochemical and physiological level) in geophyte species perform similarly, whether it is bulb, corm or tuber (Park et al. 2006; Ascough et al. 2008; Podwyszyńska 2012).
Considering all the acquired data, we postulate that CKs are heavily involved in the induction of tulip bulb formation, as in potato tuber formation (Donnelly et al. 2003; Sarkar et al. 2006; Malkawi et al. 2007). The transient increase in CK contents could promote formation of scale primordia of the future bulbs. This hypothesis is supported by indications that a specific protein transcription factor (potato homeobox1, POTH1) promotes meristem activity in potato by increasing CK levels and lowering gibberellin levels, both of which are essential for meristem development (Rodrígues-Falcón et al. 2006). The cited authors postulated that CKs may play a key role in the regulation of tuber enlargement and growth in early development stages, but not in the transduction of tuber-inducing signals. A key CK activity may be promotion of cell proliferation during very early phases of tuber growth via stimulation of cyclin D gene expression (Riou-Khamlichi et al. 1999). CKs might also modulate sink strength by activating the expression of genes involved in assimilate allocation, such as invertase, sucrose synthase, and hexose transporter genes (Roitsch and Ehness 2000). In accordance with these suggestions, and our findings, exogenous CK (BA) and low temperature also promote bulb primordia development in Lilium sp. cultured in vitro according to Ishimori et al. (2007).
O-glucosylation of particular zeatin isomers is catalysed by specific O-glucosyltransferases (Mok et al. 2005; Sakakibara 2006). Thus, since our ‘Blue Parrot’ shoots generally contained higher levels of both tZROG and cZOG (specially at S6, bulb growth initiation) than ‘Prominence’ shoots, we suggest that ‘Blue Parrot’ low bulbing ability may be related (inter alia) to relatively high O-glucosides contents, which may result from enhanced O-glucosyltransferase activity. Therefore, O-glucosides accumulated at S6 in ‘Blue Parrot’ shoots might be gradually converted to active CKs, which could retard further bulb growth.
We postulate that the more dynamic and faster accumulation of CKs in ‘Prominence’ than in ‘Blue Parrot’ could result from more active mechanisms of CK uptake, biosynthesis, transition and degradation of CKs, since rates of changes in contents of free bases, nucleosides, nucleotides and O-glucosides during successive bulb formation phases were much higher in ‘Prominence’ except of the last phase, S6. tZ CKs probably play a key role. This conclusion is supported by the effects of iP-pretreatment, including higher bulb production rates in conjunction with much stronger and earlier accumulation of tZ CKs.
Based on all the above evidence we suggest that the poor bulb formation capacity of ‘Blue Parrot’ may be related to the down regulation of tZs accumulation and severe retardation of the successive peaks in levels of iP CKs and tZs in phase S4 when bulb induction process was highly active in ‘Prominence’. Instead, the huge CK accumulation in ‘Blue Parrot’ during latest phase (S6) may have inhibited not only the bulbing processes per se, but also the associated leaf yellowing, which is known to be under CK control (Sakakibara 2006; Werner and Schmülling 2009).
To conclude, our results clearly indicate that in tulips cultured in vitro, bulbing ability is associated with changes in isoprenoid CK contents after cold treatment completion. The most pronounced changes are a transient increase in levels of tZ CKs during cold treatment and a rapid decline during bulb formation. We also postulate here that tZ-type CKs accumulate due to a gradual preceding rise in levels of iP CK metabolites.
Abbreviations
- ABA:
-
Abscisic acid
- BA:
-
Benzyladenine
- CK:
-
Cytokinin
- cZ:
-
cis-Zeatin
- cZOG:
-
cis-Zeatin-O-glucoside
- cZR:
-
cis-Zeatin-riboside
- cZRMP:
-
cis-Zeatin riboside-5′-monophosphate
- cZROG:
-
cis-Zeatin riboside-O-glucoside
- DZ:
-
Dihydrozeatin
- DZOG:
-
Dihydrozeatin-O-glucoside
- DZR:
-
Dihydrozeatin riboside
- DZRMP:
-
Dihydrozeatin riboside-5′-monophosphate
- DZROG:
-
Dihydrozeatin riboside-O-glucoside
- DW:
-
Dry weight
- IAA:
-
Indole-3-acetic acid
- iP:
-
Isopentenyladenine
- iPR:
-
Isopentenyladenosine
- iPRMP:
-
Isopentenyladenosine-5′-monophosphate
- NAA:
-
1-Naphthaleneacetic acid
- PGR:
-
Plant growth regulator
- TDZ:
-
1-Phenyl-3-(1,2,3-thiadiazol-5-yl)urea, thidiazuron
- tZ:
-
trans-Zeatin
- tZOG:
-
trans-Zeatin-O-glucoside
- tZR:
-
trans-Zeatin riboside
- tZRMP:
-
trans-Zeatin riboside-5′-monophosphate
- tZROG:
-
trans-Zeatin riboside-O-glucoside
- Z:
-
Zeatin
- Z9G:
-
Zeatin-9-glucoside
References
Aremu AO, Plačková L, Bairu MW, Novák O, Plíhalová L, Doležal K, Finnie JF, Van Staden J (2014) How does exogenously applied cytokinin type affect growth and endogenous cytokinins in micropropagated Merwilla plumbea? Plant Cell Tissue Organ Cult 1–12. doi:10.1007/s11240-014-0477-5
Ascough GD, Erwin JE, Van Staden J (2008) In vitro storage organ formation of ornamental geophytes. Hortic Rev 34:417–444
Åstot C, Doležal K, Nordström A, Wang Q, Kunkel T et al (2000) An alternative cytokinin biosynthesis pathway. Proc Natl Acad Sci USA 97:14778–14783
Bassil NV, Mok DVS, Mok MC (1993) Partial purification of a cis–trans-isomerase of zeatin from immature seeds of Phaseolus vulgaris L. Plant Physiol 102:867–872
Capelle SC, Mok DWS, Kirchner SC, Mok MC (1983) Effect of thidiazuron on cytokinin autonomy and the metabolism of N6-(∆2-isopentenyl)[8-14C]adenosine in callus tissue of Phaseolus lunatus L. Plant Physiol 73:796–802
Chow YN, Selby C, Harvey BMR (1992) Stimulation by sucrose Narcissus bulbil formation in vitro. J Hortic Sci 67:289–293
Donnelly DJ, Coleman WK, Coleman SE (2003) Potato microtuber production and performance: a review. Am J Potato Res 80:103–115
El Grari R, Backhaus RA (1987) In vitro propagation of red squill, Urginea maritime Baker. Plant Cell Tissue Organ Cult 10:65–71
Faiss M, Zalubilová J, Strnad M, Schmülling T (1997) Conditional expression of the ipt gene indicates a function for cytokinins in paracrine signalling in whole tobacco plants. Plant J 12:401–415
Ferrante A, Mensuali-Sodi A, Serra G, Tognoni F (2003) Treatment with thidiazuron for preventing leaf yellowing in cut tulips, and chrysanthemum. Acta Hort 624:357–363
Gajdošová S, Spíchal L, Kamínek M, Hoyerová K, Novák O, Dobrev PI, Galuszka P, Klíma P, Gaudinová E, Žižková E, Hanuš J, Dančák M, Trávníček B, Pešek B, Krupička M, Vaňková R, Strnad M, Motyka V (2011) Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. J Exp Bot 62:2827–2840
Guo B, Abbasi BH, Zeb A, Xu LL, Wei YH (2011) Thidiazuron: a multi-dimensional plant growth regulator. Afr J Biotechnol 10:8984–9000
Hare PD, Van Staden J (1994) Inhibitory effect of thidiazuron on the activity of cytokinin oxidase isolated from soybean callus. Plant Cell Physiol 35:1121–1125
Hirose N, Takei K, Kuroha T, Kamada-Nobusada T, Hayashi H, Sakakibra H (2008) Regulation of cytokinin biosynthesis, compartmentalization and translocation. J Exp Bot 59:75–83
Huang CL, Chang KC, Okubo H (2005) In vitro morphogenesis from pedicels of Hippeastrum × hybridum. J Fac Agr Kyushu Univ 50:27–33
Huetterman CA, Preece JE (1993) Thidiazuron: a potent cytokinin for woody plant tissue culture. Plant Cell Tissue Organ Cult 33:105–119
Hwang I, Sakakibara H (2006) Cytokinin biosynthesis and perception. Physiol Plant 126:528–538
Ishimori T, Niimi Y, Han DS (2007) Benzyladenine and low temperature promote phase transition from juvenile to vegetative adult in bulblets of Lilium × formolongi ‘White Aga’ cultured in vitro. Plant Cell Tissue Organ Cult 88:313–318
Kamínek M, Vanĕk T, Motyka V (1987) Cytokinin activities of N6-benzyladenosine derivatives hydroxylated on the side-chain phenyl ring. J Plant Growth Regul 6:113–120
Kasahara H, Takei K, Ueda N, Hishiyama S, Yamaya T, Kamiya Y, Yamaguchi S, Sakakibara H (2004) Distinct isoprenoid origins of cis and trans-zeatin biosynthesis in Arabidopsis. J Biol Chem 279:14049–14054
Kim Y-J, Hasegawa PM, Bressan RA (1981) In vitro propagation of hyacinth. HortScience 16:645–647
Kim EK, Hahn EJ, Murthy HN, Peak KY (2003) High frequency of shoot multiplication and bulblet formation of garlic in liquid cultures. Plant Cell Tissue Organ Cult 73:231–236
Kumar S, Kashyap M, Sharma DR (2005) In vitro regeneration and bulblet growth from lily bulbscale explants as affected by retardants, sucrose and irradiance. Biol Plant 49:629–632
Le Nard M, De Hertogh AA (1993) Tulipa. In: De Hertogh AA, Le Nard M (eds) The physiology of flower bulbs. Elsevier Science, Amsterdam, pp 617–682
Le Nard M, Ducommun C, Weber G, Dorion N, Bigot C (1987) Observations sur la multiplication in vitro de la tulipe (Tulipa gesneriana L.) á partir de hampes florales prélevées chez des bulbes en cours de conservation. Agronomie 7:321–329
Malkawi A, Jensen BL, Langille AR (2007) Plant hormones isolated from “Katahdin” potato plant tissues and the influence of photoperiod and temperature on their levels in relation to tuber induction. J Plant Growth Regul 26:308–317
Mirici S, Parmaksız İ, Özcan S, Sancak C, Uranbey S, Sarıhan EO, Gümüşcü A, Gürbüz B, Arslan N (2005) Efficient in vitro bulblet regeneration from immature embryos of endangered Sternbergia fischeriana. Plant Cell Tissue Organ Cult 80:239–246
Mok DWS, Mok MC (2001) Cytokinins metabolism and action. Annu Rev Plant Mol Biol 52:89–118
Mok MC, Martin RC, Dobrev PI, Vankowa R, Ho PS, Yonekura-Sakakibara K, Sakakibara H, Mok DWS (2005) Topolins and hydroxylated thidiazuron are substrates of cytokinin O-glucosyltransferase with position specificity related to receptor recognition. Plant Physiol 137:1057–1066
Mortazavi SN, Talebi SF, Naderi R-A, Sharafi Y (2011) Regulation of ethylene biosynthesis by nitric oxide and thidiazuron during postharvest of rose. J Med Plant Res 5:5177–5183
Murashige T, Skoog F (1962) A revised medium for rapid assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nhut DT (1998) Micropropagation of lily (Lilium longiflorum) via in vitro stem node and pseudo-bulblet culture. Plant Cell Rep 17:913–916
Nishiuchi Y (1980) Studies on vegetative propagation of tulip. 4. Regeneration of bulblets in bulb scale segments cultured in vitro. J Jpn Soc Hortic Sci 49:235–239
Novák O, Tarkowski P, Tarkowska D, Doležal K, Lenobel R, Strnad M (2003) Quantitative analysis of cytokinins in plants by liquid chromatography-single-quadrupole mass spectrometry. Anal Chim Acta 480:207–218
Park J-Y, Kim H-S, Youm J-W, Kim M-S, Kim K-S, Joung H, Jeon J-H (2006) Cloning of superoxide dismutase (SOD) gene of lily ‘Marcopolo’ and expression in transgenic potato. Agric Chem Biotechnol 49:1–7
Podwyszyńska M (2001) Effect of carbohydrates on shoot multiplication and bulb formation of tulip in vitro. Rocz AR Pozn CCCXXXII Ogrodn 33:119–126
Podwyszyńska M (2006a) Wpływ auksyn, etylenu i estru metylowego kwasu jasmonowego na formowanie cebul w kulturach pędów tulipana in vitro. (Effect of ethylene, auxin and methyl jasmonate on bulb formation in vitro in tulip shoot cultures). Zesz Probl Post Nauk Rol 510:461–469 (in Polish with English summary and captions)
Podwyszyńska M (2006b) Improvement of bulb formation in micropropagated tulips by treatment with NAA and paclobutrazol or ancymidol. Acta Hort 725:679–684
Podwyszyńska M (2012) The mechanisms of in vitro storage organ formation in ornamental geophytes. Flor Ornament Biotechnol Glob Sci Books 6(1):9–23
Podwyszyńska M, Marasek A (2003) Effect of thidiazuron and paclobutrazol on regeneration potential of tulip flower stalk explants in vitro and subsequent shoot multiplication. Acta Soc Bot Pol 72:181–190
Podwyszyńska M, Sochacki D (2010) Micropropagation of tulip: production of virus-free stock plants. In: Jain SM, Ochatt SJ (eds) Protocols for in vitro propagation of ornamental plants, methods in molecular biology (Springer Protocols) 589. Humana Press/Springer, New York, pp 243–256
Podwyszyńska M, Michalczuk L, Miszczak A (2004) Content of endogenous abscisic acid in tulip shoots cultured in vitro depending on micropropagation stage and genotype. Abstracts of IX International Symposium on Flower Bulbs, Niigata, Japan, 19–22, April, 2004, pp 122
Rice RD, Alderson PG, Wright NA (1983) Induction of bulbing of tulip shoots in vitro. Sci Hortic 20:377–390
Riou-Khamlichi C, Hunley R, Jacqmard A, Murray JA (1999) Cytokinin activation of Arabidopsis cell division through a D-type cyclin. Science 283:1541–1544
Rodrígues-Falcón M, Bou J, Prat S (2006) Seasonal control of tuberization in potato: conserved elements with the flowering response. Annu Rev Plant Biol 57:151–180
Roitsch T, Ehness R (2000) Regulation of source/sink relations by cytokinins. Plant Growth Regul 32:359–367
Sakakibara H (2006) Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57:431–449
Saniewski M, Mynett K (1977) The formation of parrot-like flowers in benzyladenine treated bulbs of some tulip cultivars. Acta Hort 68:151–157
Sarkar D, Pandey SK, Sharma S (2006) Cytokinins antagonize the jasmonates action on the regulation of potato (Solanum tuberosum) tuber formation in vitro. Plant Cell Tissue Organ Cult 87:285–295
Schmitz RY, Skoog F, Playtis AJ, Leonard NJ (1972) Cytokinins: synthesis and biological activity of geometric and position isomers of zeatin. Plant Physiol 50:702–705
Šmehilová M, Galuszka P, Bilyeu KD, Jaworek P, Kowalska M, Šebela M, Sedlarova M, English JT, Frébort I (2009) Subcellular localization and biochemical comparison of cytosolic and secreted cytokinin dehydrogenase enzymes from maize. J Exp Bot 60:2701–2712
Spíchal L, Rakova NY, Riefler M, Mizuno T, Romanov GA, Strnad M, Schmülling T (2004) Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay. Plant Cell Physiol 45:1299–1305
Taeb AG, Alderson PG (1990) Shoot production and bulbing of tulip in vitro related to ethylene. J Hortic Sci 65:199–204
Takei K, Yamaya T, Sakakibara H (2004) Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin. J Biol Chem 279:41866–41872
Tarkowská D, Filek M, Biesaga-Kościelniak J, Marcińska I, Macháčková I, Krekule J, Strnad M (2012) Cytokinins in shoot apices of plants during vernalization. Plant Sci 187:105–112
Van Aartrijk J, Blom-Barnhoorn GJ (1981) Growth regulator requirements for adventitious regeneration from Lilium bulb-scale tissue in vitro, in relation to duration of bulb storage and cultivar. Sci Hortic 14:193–197
Van Scheepen J (1996) Classified list and international register of tulip names. KAVB, Hillegom
Werner T, Schmülling T (2009) Cytokinin action in plant development. Curr Opin Plant Biol 12:527–538
Zatloukal M, Gemrotov M, Doležal K, Havlíček L, Spíchal L, Strnad M (2008) Novel potent inhibitors of A. thaliana cytokinin oxidase/dehydrogenase. Bioorg Med Chem 16:9268–9275
Acknowledgments
This study was funded in part by the Polish Ministry of Scientific Research and Information Technology, Project PBZ MIN-007/P04/2003. Support from the Centre of the Region Haná for Biotechnological and Agricultural Research (Grant LO1204 from the National Program of Sustainability I) is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Podwyszyńska, M., Novák, O., Doležal, K. et al. Endogenous cytokinin dynamics in micropropagated tulips during bulb formation process influenced by TDZ and iP pretreatment. Plant Cell Tiss Organ Cult 119, 331–346 (2014). https://doi.org/10.1007/s11240-014-0537-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0537-x