Abstract
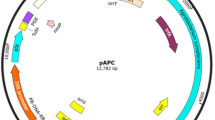
We have developed a reproducible method of Agrobacterium tumefaciens mediated stable genetic transformation of white jute (Corchorus capsularis cv. JRC 321) utilizing the shoot organogenesis potential of the shoot tip apical meristem. A. tumefaciens strain LBA4404 harboring the binary vector pCAMBIA 1301 was used in the transformation experiments. The explants were subjected to varying durations of preculture and cocultivation with A. tumefaciens in the presence of acetosyringone in order to optimize the conditions conducive for the highest expression of transgene. A schedule of 1 day preculture of shoot tips followed by 3 days cocultivation was optimized for Agrobacterium mediated stable genetic transformation of C. capsularis cv. JRC 321. The optimized lethal doses of the antibiotic hygromycin B for shoot tips (12 mg/L) and for 5 days old seedlings (14 mg/L) were employed in efficient selection of the transformed tissues. This method of transformation resulted in a mean transformation efficiency of 4.09 %. Stable expression of the intron harbored gusA transgene was observed in mature organs of the transformed plants and their progenies. Genomic integration and inheritance of the hpt transgene was further confirmed by Southern blot analysis. The transformed plants exhibited normal morphology and most of them produced viable progenies, many of which segregated in a 3:1 ratio following Mendelian inheritance for a single dominant locus. However, strong P value support for 3:1 segregation ratio was obtained in case of two lines of independent transformants. Nevertheless, the method of transformation mentioned in this protocol could be effectively implemented in genetic transformation of many other cultivars of jute due to the genotype independent regeneration potential of the shoot tip explants.






Similar content being viewed by others
Abbreviations
- ACM:
-
Agrobacterium culture medium
- BAP:
-
N6-Benzylaminopurine
- BM:
-
Basal medium
- CCM:
-
Cocultivation medium
- GA3 :
-
Gibberellic acid
- GM:
-
Germination medium
- GUS:
-
β-Glucuronidase protein
- gusA :
-
β-Glucuronidase gene
- hpt :
-
Hygromycin phosphotransferase gene
- IAA:
-
Indole-3-acetic acid
- IBA:
-
Indole-3-butyric acid
- LB:
-
Luria Bertani
- MS:
-
Murashige and Skoog (1962)
- OD600 :
-
Optical density at a wavelength of 600 nm
- PCM:
-
Preculture medium
- PCR:
-
Polymerase chain reaction
- RM:
-
Rooting medium
- SEM:
-
Shoot elongation medium
- SGM:
-
Shoot growth medium
- SM:
-
Shooting medium
- vir :
-
Virulence genes of Agrobacterium tumefaciens
References
Amin MN, Khatun A, Bhuiyan MSR, Sayed MA, Khandker SR (2012) Genetic transformation in white jute through Agrobacterium and salinity screening of transgenic plant. Bangladesh J Agric Res 37:97–107
Aragao FJL, Sarokin L, Vianna GR, Rech EL (2000) Selection of transgenic meristematic cells utilizing a herbicidal molecule results in the recovery of fertile transgenic soybean (Glycine max L. Merril) plants at high frequency. Theor Appl Genet 101:1–6
Bharadwaj P, Beena MR, Sinha MK, Kirti PB (2011) In vitro regeneration and optimization of conditions for Agrobacterium mediated transformation in jute, Corchorus capsularis. J Plant Biochem Biotechnol 20:39–46
Birch RG (1997) Plant transformation: problems and strategies for practical application. Annu Rev Plant Physiol Plant Mol Biol 48:297–326
Chattopadhyay T, Roy S, Mitra A, Maiti MK (2010) Development of a transgenic hairy root system in jute (Corchorus capsularis L.) with gusA reporter gene through Agrobacterium rhizogenes mediated co-transformation. Plant Cell Rep 30:485–493
Christou P, McCabe DE, Martinell BJ, Swain WF (1990) Soybean genetic engineering—commercial production of transgenic plants. Trends Biotechnol 8:145–151
Datta SK, Peterhans A, Datta K, Potrykus I (1990) Genetically engineered fertile Indica rice recovered from protoplasts. Nat Biotechnol 8:736–740
Datta K, Vasquez A, Tu J, Torrizo L, Alam MF, Oliva N, Abrigo E, Khush GS, Datta SK (1998) Constitutive and tissue-specific differential expression of cryIA(b) gene in transgenic rice plants conferring resistance to rice insect pest. Theor Appl Genet 97:20–30
Devi PB, Sticklen MB (2003) In vitro culture and genetic transformation of sorghum by microprojectile bombardment. Plant Biosyst 137:249–254
Dutt M, Li ZT, Dhekney SA, Gray DJ (2007) Transgenic plants from shoot apical meristems of Vitis vinifera L. ‘‘Thompson Seedless’’ via Agrobacterium-mediated transformation. Plant Cell Rep 26:2101–2110
Edmonds JM (1990) Herbarium survey of African Corchorus L. species. International Board for Plant Genetic Resources, Rome
Ghosh M, Saha T, Nayak P, Sen SK (2002) Genetic transformation by particle bombardment of cultivated jute, Corchorus capsularis L. Plant Cell Rep 20:936–942
Gordon-Kamm WJ, Spencer TM, Mangano ML et al (1990) Transformation of maize cells and regeneration of fertile transgenic plants. Plant Cell 2:603–618
Gould J, Magallanes-Cedeno M (1998) Adaptation of cotton shoot apex culture to Agrobacterium mediated transformation. Plant Mol Biol Rep 16:1–10
Hansen G, Wright MS (1999) Recent advances in transformation of plants. Trends Plant Sci 4:226–231
Hossain ABM, Ahmed G, Khan RI, Islam MS (1998) Transient GUS expression in jute (Corchorus capsularis L. var. D-154) explants after infection by Agrobacterium tumefaciens. Plant Tissue Cult 8:11–18
Islam MR, Khan MH, Zohra FT, Hossain MB, Seraj ZI (1999) Stable transformation of jute (Corchorus capsularis L. var. CVL-1) calli and high frequency marker gene insertion in explants. Plant Tissue Cult 9:35–43
Islam MS, Huda KMK, Mahmud F, Banu SA, Wang MH (2009) Regeneration and genetic transformation of Tossa Jute (Corchorus olitorius L.). Aust J Crop Sci 3:287–293
Jefferson RA, Kavanagh TA, Bevan MW (1987) GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6:3901–3907
Khatun A, Saha CK, Naher Z, Mahbub S, Siddique AB, Bilkis S (2003) Plant regeneration from the cotyledons of tossa jute (Corchorus olitorius L.). Biotechnology 2:206–213
Ko K, Brown SK, Norelli JL, Aldwinckle HS (1998) Alterations in nptII and gus expression following micropropagation of transgenic M.7 apple rootstock lines. J Am Soc Hortic Sci 123:11–18
Kundu BC, Rao NS (1954) Origin and development of axillary buds in jute (Corchorus capsularis). Ann Bot 18:367–375
Kundu A, Sarkar D, Bhattacharjee A, Topdar N, Sinha MK, Mahapatra BS (2011) A simple ethanol wash of the tissue homogenates recovers high-quality genomic DNA from Corchorus species characterized by highly acidic and proteinaceous mucilages. Electron J Biotechnol 14:1. doi:10.2225/vol14-issue1-fulltext-4
Liu HK, Yang C, Wei ZM (2004) Efficient Agrobacterium tumefaciens mediated transformation of soybeans using an embryonic tip regeneration system. Planta 219:1042–1049
Mishiba KI, Chin DP, Mii M (2005) Agrobacterium-mediated transformation of Phalaenopsis by targeting protocorms at an early stage after germination. Plant Cell Rep 24:297–303
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15:473–497
Naher Z, Khatun A, Mahbub S, Alim MA, Siddique AS (2003) Influence of genotypes on plant regeneration from cotyledons of Corchorus capsularis L. Biotechnology 2:44–51
Olhoft PM, Flagel LE, Donovan CM, Somers DA (2003) Efficient soybean transformation using hygromycin B selection in the cotyledonary-node method. Planta 216:723–735
Patel GI, Datta RM (1960) Interspecific hybridization between Corchorus olitorius and C. capsularis and the cytogenetical basis of incompatibility between them. Euphytica 9:89–110
Roy A, Bandyopadhyay A, Mahaptra AK, Ghosh SK, Singh NK, Bansal KC, Koundal KR, Mohapatra T (2006) Evaluation of genetic diversity in jute (Corchorus species) using STMS, ISSR and RAPD markers. Plant Breed 125:292–297
Saha P, Sarkar D, Kundu A, Majumder S, Datta SK, Datta K (2014) Karyotype analysis and chromosomal evolution in Asian species of Corchorus (Malvaceae s.l.). Genet Resour Crop Evol. doi: 10.1007/s10722-014-0099-0
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, New York
Sarker RH, Al-Amin GM, Hassan F, Hoque MI (2008) Agrobacterium-mediated genetic transformation of two varieties of jute (Corchorus capsularis L.). Plant Tissue Cult Biotechnol 18:7–16
Sheng J, Clitovsky V (1996) Agrobacterium-plant cell DNA transport: have virulence proteins, will travel. Plant Cell 8:1699–1710
Sticklen MB, Oraby HF (2005) Shoot apical meristem: a sustainable explant for genetic transformation of cereal crops. In Vitro Cell Dev Biol Plant 41:187–200
Swaminathan MS, Iyer RD, Sulbha K (1961) Morphology, cytology and breeding behavior of hybrids between Corchorus olitorius and C. capsularis. Curr Sci 30:67–68
Villemont E, Dubois F, Sangwan RS, Vasseur G, Bourgeois Y, Sangwan-Norrel BS (1997) Role of the host cell cycle in the Agrobacterium-mediated genetic transformation of Petunia: evidence of an S-phase control mechanism for T-DNA transfer. Planta 201:160–172
Wang QC, Valkonen JPT (2008) Elimination of two viruses which act synergistically from sweet potato by shoot tip culture and cryotherapy. J Virol Methods 154:135–145
Wilmink A, Dons JJM (1993) Selective agents and marker genes for use in transformation of monocotyledonous plant. Plant Mol Biol Rep 11:165–185
Yoshida S, Forno DA, Cock JH, Gomez KA (1976) Routine procedures for growing rice plants in culture solutions. Laboratory manual for physiological studies of rice. International Rice Research Institute, Los Banos, pp 61–66
Zapata C, Srivatanakul M, Park SH, Lee BM, Salas MG, Smith RH (1999) Improvements in shoot apex regeneration of two fiber crops: cotton and kenaf. Plant Cell Tissue Organ Cult 56:185–191
Zhang K, Wang J, Hu X, Yang A, Zhang J (2010) Agrobacterium-mediated transformation of shoot apices of Kentucky bluegrass (Poa pratensis L.) and production of transgenic plants carrying a betA gene. Plant Cell Tissue Organ Cult 102:135–143
Zhong H, Sun B, Warkentin D, Zhang S, Wu R, Wu T, Sticklen MB (1996) The competence of maize shoot meristems for integrative transformation and inherited expression of transgenes. Plant Physiol 110:1097–1107
Acknowledgments
We thank University Grants Commission (UGC) for funding in the form of UGC/UPE scheme at university of Calcutta, Department of Biotechnology (DBT), Government of India and RKVY project under Government of West Bengal, and Ministry of Agriculture, Government of India. Comments and suggestions on the manuscript from two anonymous reviewers are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saha, P., Datta, K., Majumder, S. et al. Agrobacterium mediated genetic transformation of commercial jute cultivar Corchorus capsularis cv. JRC 321 using shoot tip explants. Plant Cell Tiss Organ Cult 118, 313–326 (2014). https://doi.org/10.1007/s11240-014-0484-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0484-6