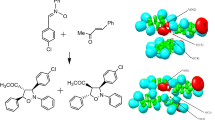
Abstract
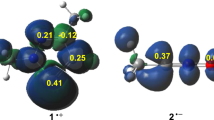
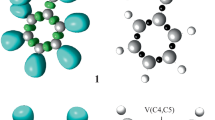
The intramolecular [3 + 2] cycloaddition (IM32CA) reactions of azides and diazoalkanes leading to fused tricyclic 1,2,3-triazolines and 1-pyrazolines have been studied at the MPWB1K/6-311G(d,p) computational level within the molecular electron density theory (MEDT). The IM32CA reactions of azides are classified as zw- type following non-concerted one-step mechanism with earlier N1-C5 bond formation implied from the bonding evolution theory (BET) study with the Gibbs free activation energies between 26.3–30.0 kcal mol−1 at 298 K in benzene while that of the diazoalkanes are classified as pmr- type with the earlier C3-C5 bond formation and Gibbs free activation energies between 24.7–29.1 kcal mol−1 at 298 K in n-pentane. The influence of substituent effects on these IM32CA reactions is studied. Azide reactions with minimal global electron density transfer (GEDT) are classified as the null electron density flux (NEDF) while that of the diazoalkanes are classified as forward electron density flux (FEDF). QTAIM analysis and ELF study allow revealing the non-covalent interactions at the TSs.












Similar content being viewed by others
Availability of data and materials
Data and materials are available from the corresponding author on request.
References
Obaid RJ, Mughal EU, Naeem N, Al-Rooqi MM, Sadiq A, Jassas RS, Moussa Z, Ahmed SA (2022) Pharmacological significance of nitrogen-containing five and six-membered heterocyclic scaffolds as potent cholinesterase inhibitors for drug discovery. Process Biochem 120:250–259. https://doi.org/10.1016/j.procbio.2022.06.009
Jampilek J (2019) Heterocycles in medicinal chemistry. Molecules 24:3839. https://doi.org/10.3390/molecules24213839
Heravi MM, Zadsirjana V (2020) Prescribed drugs containing nitrogen heterocycles: an overview. RSC Adv 10:44247–44311. https://doi.org/10.1039/D0RA09198G
Frühauf A, Behringer M, Meyer-Almes F-J (2023) Significance of five-membered heterocycles in human histone deacetylase inhibitors. Molecules 28:5686. https://doi.org/10.3390/molecules28155686
Kadaba PK (1988) Triazolines. 14. 1,2,3-Triazolines and triazoles. A new class of anticonvulsants. Drug design and structure-activity relationships. J Med Chem 31:196–203. https://doi.org/10.1021/jm00396a032
Boshra AN, Abdu-Allah HHM, Mohammed AF, Hayallah AM (2020) Click chemistry synthesis, biological evaluation and docking study of some novel 2’-hydroxychalcone-triazole hybrids as potent anti-inflammatory agents. Bioorg Chem 95:103505. https://doi.org/10.1016/j.bioorg.2019.103505
Soltis MJ, Yeh HJ, Cole KA, Whittaker N, Wersto RP, Kohn EC (1996) Identification and characterization of human metabolites of CAI [5-amino-1-1(4’-chlorobenzoyl-3,5-dichlorobenzyl)-1,2,3-triazole- 4-carboxamide). Drug Metab Dispos 24:799–806
Neu HC, Fu KP (1979) Cefatrizine activity compared with that of other cephalosporins. Antimicrob Agents Chemother 15:209–212. https://doi.org/10.1128/AAC.15.2.209
Higashitani F, Hyodo A, Ishida N, Inoue M, Mitsuhashi S (1990) Inhibition of betalactamases by tazobactam and in-vitro antibacterial activity of tazobactam combined with piperacillin. J Antimicrob Chemother 25:567–574. https://doi.org/10.1093/jac/25.4.567
Yadav N, Agarwal D, Kumar S, Dixit AK, Gupta RD, Awasthi SK (2018) In vitro antiplasmodial efficacy of synthetic coumarin-triazole analogs. Eur J Med Chem 145:735–745. https://doi.org/10.1016/j.ejmech.2018.01.017
Zhang C, Li N, Niu F (2019) Baicalein triazole prevents respiratory tract infection by RSV through suppression of oxidative damage. Microb Pathog 131:227–233. https://doi.org/10.1016/j.micpath.2019.03.026
Shaaban MR, Mayhoub AS, Farag AM (2012) Recent advances in the therapeutic applications of pyrazolines. Expert Opin Ther Pat 22:253–291. https://doi.org/10.1517/13543776.2012.667403
Ahsan MJ, Ali A, Ali A, Thiriveedhi AK, Bakht MA, Yusuf M, Afzal O, Altamimi ASA (2022) Pyrazoline containing compounds as therapeutic targets for neurodegenerative disorders. ACS Omega 7:38207–38245. https://doi.org/10.1021/acsomega.2c05339
Tok F, Abas BI, Cevik O, Kaymakcioglu BK (2020) Design, synthesis and biological evaluation of some new 2-Pyrazoline derivatives as potential anticancer agents. Bioorg Chem 102:104063. https://doi.org/10.1016/j.bioorg.2020.104063
Ahsan MJ (2012) Synthesis and anticancer activity of 3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide/ carbothioamide analogues. Lett Drug Des Dis 9:823–827. https://doi.org/10.2174/157018012803307996
Ahsan MJ, Khalilullah H, Stables JP, Govindasamy J (2013) Synthesis and anticonvulsant activity of 3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide/carbothioamide analogues. J Enzy Inh Med Chem 28:644–650. https://doi.org/10.3109/14756366.2012.663364
Ahsan MJ (2017) Anticonvulsant activity and neuroprotection assay of 3-substituted-N-aryl-6,7-dimethoxy-3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide analogues. Arab J Chem 10:S2762–S2766. https://doi.org/10.1016/j.arabjc.2013.10.023
Akhtar M, Marella A, Alam MM, Khan MF, Akhtar M, Anwer T, Khan F, Naematullah M, Azam F, Rizvi MA, Shaquiquzzaman M (2020) Design and synthesis of pyrazole–pyrazoline hybrids as cancer-associated selective COX-2 inhibitors. Arch Pharm 2020:e2000116. https://doi.org/10.1002/ardp.202000116
Kumar G, Tanwar O, Kumar J, Akhter M, Sharma S, Pillai CR, Alam MM, Zama MS (2018) Pyrazole-pyrazoline as promising novel antimalarial agents: a mechanistic study. Eur J Med Chem 149:139–147. https://doi.org/10.1016/j.ejmech.2018.01.082
Ahsan MJ, Govindasamy J, Khalilullah H, Mohan G, Stables JP, Pannecouque C, Clercq ED (2012) POMA analyses as new efficient bioinformatics’ platform to predict and optimize bioactivity of synthesized 3a,4-dihydro-3H-indeno[1,2-c]pyrazole-2-carboxamide/carbothioamide analogues. Bioorg Med Chem Lett 22:7029–7035. https://doi.org/10.1016/j.bmcl.2012.09.108
Breugst M, Reissig HU (2020) The huisgen reaction: milestones of the 1,3- dipolar cycloaddition. Angew Chem 59:12293–12307. https://doi.org/10.1002/anie.202003115
Fusco R, Garanti L, Zecchi G (1975) Intramolecular 1,3-dipolar cycloadditions of aryl azides bearing alkenyl, alkynyl, and nitrile groups. J Org Chem 40:1906–1909. https://doi.org/10.1021/jo00901a007
Kirmse W, Dietrich H (1967) Reactions of 2-allyloxy-phenyldiazomethane. Chem Ber 100:2710–2718
Miguel ID, Velado M, Herradón B, Mann E (2016) Synthetic studies on the application of the intramolecular azide-alkene 1,3-dipolar cycloaddition reaction in the construction of the core structure of complex alkaloids. Tetrahedron 72:4617–4625. https://doi.org/10.1016/j.tet.2016.06.023
Repetto E, Oliveira Udry GA, Varela O (2019) Intramolecular azide-alkene cycloaddition-elimination reaction in an aldohex-2-enonic acid derivative. Carbohydr Res 483:107751. https://doi.org/10.1016/j.carres.2019.107751
Fryźlewicz A, Kącka-Zych A, Demchuk OM, Mirosław B, Woliński P, Jasiński R (2021) Green synthesis of nitrocyclopropane-type precursors of inhibitors for the maturation of fruits and vegetables via domino reactions of diazoalkanes with 2-nitroprop-1-ene. J Clean Prod 292:126079. https://doi.org/10.1016/j.jclepro.2021.126079
Fukui K, Yonezawa T, Shingu H (1952) A molecular orbital theory of reactivity in aromatic hydrocarbons. J Chem Phys 20:722–725. https://doi.org/10.1063/1.1700523
Ess DH, Houk KN (2007) Distortion/interaction energy control of 1,3-dipolar cycloaddition reactivity. J Am Chem Soc 129:10646–10647. https://doi.org/10.1021/ja0734086
Domingo LR (2016) Molecular electron density theory: a modern view of reactivity in organic chemistry. Molecules 21:1319. https://doi.org/10.3390/molecules21101319
Domingo LR, Acharjee N (2020) In: Ul-Haq Z, Wilson AK (ed) Molecular electron density theory: a new theoretical outlook on organic chemistry. Frontiers in Computational Chemistry 174–227. https://doi.org/10.2174/9789811457791120050007
Ríos-Gutiérrez M, Domingo LR (2019) Unravelling the mysteries of the [3+2] cycloaddition reactions. Eur J Org Chem 2019:267–282. https://doi.org/10.1002/ejoc.201800916
Domingo LR, Gutiérrez MR, Pérez P (2018) a molecular electron density theory study of the reactivity and selectivities in [3 + 2] cycloaddition reactions of C, N-dialkyl nitrones with ethylene derivatives. J Org Chem 83:2182–2197. https://doi.org/10.1021/acs.joc.7b03093
Acharjee N, Mohammad Salim HA, Chakraborty M (2021) Unveiling [3 + 2] cycloaddition reactions of benzonitrile oxide and diphenyl diazomethane to cyclopentene and norbornene: a molecular electron density theory perspective. Theor Chem Acc 140:113. https://doi.org/10.1007/s00214-021-02811-3
Domingo LR, Acharjee N (2021) Unveiling the chemo- and regioselectivity of the [3+2] cycloaddition reaction between 4-chlorobenzonitrile oxide and β-aminocinnamonitrile with a MEDT perspective. ChemistrySelect 6:4521–4532. https://doi.org/10.1002/slct.202100978
Domingo LR, Ríos-Gutiérrez M, Acharjee N (2019) A molecular electron density theory study of the chemoselectivity, regioselectivity, and diastereofacial selectivity in the synthesis of an anticancer spiroisoxazoline derived from α-santonin. Molecules 24:832. https://doi.org/10.3390/molecules24050832
Acharjee N, Mohammad Salim HA, Chakraborty M, Rao MP, Ganesh M (2021) Unveiling the high regioselectivity and stereoselectivity within the synthesis of spirooxindolenitropyrrolidine: a molecular electron density theory perspective. J Phys Org Chem 34:e4189. https://doi.org/10.1002/poc.4189
Domingo LR, Ríos-Gutiérrez M, Acharjee N (2022) A molecular electron density theory study of the Lewis acid Catalyzed [3+2] Cycloaddition reactions of nitrones with nucleophilic ethylenes. Eur J Org Chem. https://doi.org/10.1002/ejoc.202101417
Domingo LR, Gutiérrez MR, Pérez P (2018) A molecular electron density theory study of the role of the copper metalation of azomethine ylides in [3 + 2] cycloaddition reactions. J Org Chem 83:10959–10973. https://doi.org/10.1021/acs.joc.8b01605
Domingo LR, Acharjee N (2020) Unravelling the strain-promoted [3+2] cycloaddition reactions of phenyl azide with cycloalkynes from the molecular electron density theory perspective. New J Chem 44:13633–13643. https://doi.org/10.1039/D0NJ02711A
Domingo LR, Acharjee N (2018) [3+2] Cycloaddition reaction of C-phenyl-N-methyl nitrone to acyclic-olefin-bearing electron-donating substituent: a molecular electron density theory study. ChemistrySelect 3:8373–8380. https://doi.org/10.1002/slct.201801528
Domingo LR, Acharjee N (2021) Unveiling the substituent effects in the stereochemistry of [3+2] cycloaddition reactions of aryl- and alkyldiazomethylphosphonates with norbornadiene within a MEDT perspective. ChemistrySelect 6:10722–10733. https://doi.org/10.1002/slct.202102942
Acharjee N, Mondal A, Chakraborty M (2022) Unveiling the intramolecular [3 + 2] cycloaddition reactions of C, N-disubstituted nitrones from the molecular electron density theory perspective. New J Chem 46:7721–7733. https://doi.org/10.1039/d2nj00888b
Mondal A, Domingo LR, Acharjee N (2023) Revealing the influence of tether length on the intramolecular [3 + 2] cycloaddition reactions of nitrones from the molecular electron density theory perspective. J Phys Org Chem 2023:e4574. https://doi.org/10.1002/poc.4574
Becke AD, Edgecombe KE (1990) A simple measure of electron localization in atomic and molecular systems. J Chem Phys 92:5397. https://doi.org/10.1063/1.458517
Silvi B, Savin A (1994) Classification of chemical bonds based on topological analysis of electron localization functions. Nature 371:683–686. https://doi.org/10.1038/371683a0
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516. https://doi.org/10.1021/ja00364a005
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels-Alder reactions. Tetrahedron 58:4417–4423. https://doi.org/10.1016/S0040-4020(02)00410-6
Krokidis X, Noury S, Silvi B (1997) Characterization of elementary chemical processes by catastrophe theory. J Phys Chem A 101:7277–7282. https://doi.org/10.1021/jp9711508
Thom R (1972) Stabilité structurelle et morphogénèse. Intereditions, Paris
Bader RFW (1994) Atoms in molecules: a quantum theory. Oxford University Press, Oxford, New York
Bader RFW, Essén H (1984) The characterization of atomic interactions. J Chem Phys 80:1943. https://doi.org/10.1063/1.446956
García JC, Johnson ER, Keinan S, Chaudret R, Piquemal JP, Beratan DN, Yang W (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7:625–632. https://doi.org/10.1021/ct100641a
Domingo LR, Chamorro E, Perez P (2010) Understanding the high reactivity of the azomethine ylides in [3+2] cycloaddition reactions. Lett Org Chem 7:432–439. https://doi.org/10.2174/157017810791824900
Domingo LR, Ríos-Gutiérrez M (2017) A molecular electron density theory study of the reactivity of azomethine imine in [3+2] cycloaddition reactions. Molecules 22:750. https://doi.org/10.3390/molecules22050750
Ríos-Gutiérrez M, Domingo LR (2019) The carbenoid-type reactivity of simplest nitrile imine from a molecular electron density theory perspective. Tetrahedron 75:1961–1967. https://doi.org/10.1016/j.tet.2019.02.014
Parr RG, Szentpály LV, Liu S (1999) Electrophilicity Index. J Am Chem Soc 121:1922–1924. https://doi.org/10.1021/ja983494x
Domingo LR, Pérez P (2011) The nucleophilicity N index in organic chemistry. Org Biomol Chem 9:7168–7175. https://doi.org/10.1039/C1OB05856H
Bastide J, Hamelin J, Texier F, Vo Quang Y (1973) Bull Soc Chim. France: 2555
Jasiński R (2015) A stepwise, zwitterionic mechanism for the 1,3-dipolar cycloaddition between (Z)-C-4-methoxyphenyl-N phenylnitrone and gem-chloronitroethene catalysed by 1-butyl-3-methylimidazolium ionic liquid cations. Tetrahedron Lett 56:532–535. https://doi.org/10.1016/j.tetlet.2014.12.007
Domingo LR (2014) A new C-C bond formation model based on the quantum chemical topology of electron density. RSC Adv 4:32415–32428. https://doi.org/10.1039/C4RA04280H
Domingo LR, Ríos-Gutiérrez M, Perez P (2020) A molecular electron density theory study of the participation of tetrazines in aza-Diels–Alder reactions. RSC Adv 10:15394–15405. https://doi.org/10.1039/D0RA01548B
Domingo LR, Ríos-Gutiérrez M (2023) A useful classification of organic reactions based on the flux of the electron density. Sci Rad 2:1–24. https://doi.org/10.58332/scirad2023v2i1a01
Espinosa E, Alkorta I, Elguero J, Molins E (2002) From weak to strong interactions: a comprehensive analysis of the topological and energetic properties of the electron density distribution involving X-H⋯F–Y systems. J Chem Phys 117:5529–5542. https://doi.org/10.1063/1.1501133
Emamian S, Soleymani M, Moosavi SS (2019) Copper(I)-catalyzed asymmetric aza Diels-Alder reactions of azoalkenes toward fulvenes: a molecular electron density theory study. New J Chem 43:4765–4776. https://doi.org/10.1039/C9NJ00269C
Emamian S, Lu T, Kruse H, Emamian H (2019) Exploring nature and predicting strength of hydrogen bonds: a correlation analysis between atoms-in-molecules descriptors, binding energies, and energy components of symmetry-adapted perturbation theory. J Comput Chem 40:2868–2881. https://doi.org/10.1002/jcc.26068
Acknowledgements
Asmita Mondal acknowledges the support of University Grants Commission (UGC), Government of India, for Savitribai Jyotirao Phule Single Girl Child (SJSGC) Fellowship.
Funding
No funding received.
Author information
Authors and Affiliations
Contributions
AM performed the calculations and wrote the first draft of the manuscript. All figures, tables and schemes were then reviewed by HAMS and MC. The complete manuscript was then reviewed and finalized by NA.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11224_2023_2270_MOESM1_ESM.doc
(1) Computational Methods (2) BET study of the IM32CA reaction of azide 1 (3) BET study of the IM32CA reaction of diazoalkane 10 (4) References (5) Table with the MPWB1K/6-311G(d,p) calculated electronic energies (au) in gas phase and benzene of the stationary points involved in the IM32CA reactions of azides 1-3,12-13 and diazoalkanes 10,16-19 (6) Table with the MPWB1K/6-311G(d,p) calculated enthalpies (au), entropies (Calmol-1K-1) and Gibbs free energies (au) of the stationary points involved in the IM32CA reactions of azides 1-3,12-13 computed at 353 K and 298K in benzene and diazoalkanes 10,16-19 computed at 298 K in n-pentane (7) MPWB1K/6-311G(d,p) gas phase computed Cartesian coordinates of the stationary points involved in the IM32CA reactions of azides 1-3,12-13 (8) MPWB1K/6-311G(d,p) computed Cartesian coordinates of the stationary points involved in the IM32CA reactions of azides 1-3,12-13 in benzene at 353K (9) MPWB1K/6-311G(d,p) gas phase computed Cartesian coordinates of the stationary points involved in the IM32CA reactions of azides 1-3,12-13 in benzene at 298K (10) MPWB1K/6-311G(d,p) gas phase computed Cartesian coordinates of the stationary points involved in the IM32CA reactions of diazoalkanes 10,16-19 (11) MPWB1K/6-311G(d,p) computed Cartesian coordinates of the stationary points involved in the IM32CA reactions of diazoalkanes 10,16-19 in n-pentane at 298K. (DOC 473 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mondal, A., Acharjee, N., Mohammad-Salim, H.A. et al. A molecular electron density theory study to understand intramolecular [3 + 2] cycloaddition reactions of azides and diazoalkanes. Struct Chem (2024). https://doi.org/10.1007/s11224-023-02270-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11224-023-02270-5