Abstract
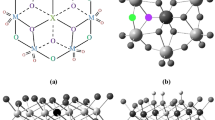
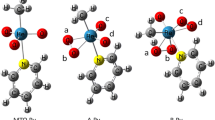
In this work, the geometric and electronic parameters of pure and vanadium-substituted anions of Lindqvist polyoxometalates have been investigated using DFT calculations. Active sites of all anions are identified through results on local and global reactivity descriptors. The results indicate that bridging oxygen atoms in all clusters are the most active sites. Using this, the mechanism of the catalytic generation of the hydroxyl radical from water was studied for the di-substituted Lindqvist polyoxometalate [V2Mo4O19]4−. This study provides a detailed understanding of this important intermediate step in photo-oxidation reactions. The mechanistic route makes it possible to locate transition states and intermediates structures and demonstrates that the pre-association of the water molecule leads to an H abstraction with an energy barrier of 26.42 kcal/mol and OH radical and [HV2Mo4O19]4− as products in this step.







Similar content being viewed by others
Availability of data and materials
All data generated or analysed during this study are included in this published article.
References
Modak B, Srinivasu K, Ghosh SK (2014) A hybrid DFT based investigation of the photocatalytic activity of cation–anion codoped srtio3 for water splitting under Visible light. Phys Chem Chem Phys. https://doi.org/10.1039/c4cp02856b
Ammam M (2013) Polyoxometalates: Formation, structures, principal properties, main deposition methods and application in sensing. J Mater Chem A. https://doi.org/10.1039/c3ta01663c
López X, Carbó JJ, Bo C, Poblet JM (2012) Structure, properties and reactivity of polyoxometalates: a theoretical perspective. Chem Soc Rev. https://doi.org/10.1039/c2cs35168d
Huang B, Yang D-H, Han B-H (2020) Application of polyoxometalate derivatives in rechargeable batteries. J Mater Chem A. https://doi.org/10.1039/c9ta12679a
Almi M, Zhou M, Saal A, Springborg M (2023) Mechanistic insights into aerobic oxidative cleavage of glycol catalyzed by an Anderson-type polyoxometalate [IMO6O24]5−. J Mol Model. https://doi.org/10.1007/s00894-023-05458-y
Diaz-Uribe CE, Rodríguez A, Utria D, Vallejo W, Puello E, Zarate X, Schott E (2018) Photocatalytic degradation of methylene blue by the Anderson-type polyoxomolybdates/tio2 thin films. Polyhedron. https://doi.org/10.1016/j.poly.2018.04.027
Wang Y, Shi L, Qian B, Huang X, Sun X, Wang B, Song XM, Huang H, Zhang Y, Ma T (2021) Regeneratable trinuclear iron-OXO cocatalyst for photocatalytic hydrogen evolution. Chem Eng J. https://doi.org/10.1016/j.cej.2020.127551
Zhang Y, Liu J, Li SL, Su ZM, Lan YQ (2019) Polyoxometalate-based materials for sustainable and clean energy conversion and storage. Energy Chem. https://doi.org/10.1016/j.enchem.2019.100021
Streb C (2012) New trends in polyoxometalate photoredox chemistry: From photosensitisation to water oxidation catalysis. Dalton Trans. https://doi.org/10.1039/c1dt11220A
Forster J, Rösner B, Khusniyarov MM, Streb C (2011) Tuning the light absorption of a molecular vanadium oxide system for enhanced photooxidation performance. Chem Commun. https://doi.org/10.1039/c0cc05536k
Ying-Jie H, Xiao-Nan X, Xiao-Mei Z, Lei-Yun H, Meng W, Chong-Tai W (2014) A study on the visible photocatalytic property of PW11O39Co(II)(H2O)5−in homogeneous and heterogeneous systems. Chin J Inorg Chem. https://doi.org/10.11862/CJIC.2014.250
Hua Y, Chen G, Xu X, Zou X, Liu J, Wang B, Zhao Z, Chen Y, Wang C, Liu X (2014) Comparative study of homogeneous and heterogeneous photocatalytic degradation of RHB under visible light irradiation with Keggin-type manganese-substituted catalysts. J Phys Chem C. https://doi.org/10.1021/jp409082q
Tucher J, Wu Y, Nye LC, Ivanovic-Burmazovic I, Khusniyarov MM, Streb C (2012) Metal substitution in a Lindqvist polyoxometalate leads to improved photocatalytic performance. Dalton Trans. https://doi.org/10.1039/c2dt30304c
Tucher J, Schlicht S, Kollhoff F, Streb C (2014) Photocatalytic reactivity tuning by heterometal and Addenda Metal Variation in Lindqvist polyoxometalates. Dalton Trans. https://doi.org/10.1039/c4dt02682a
Hua Y, Wang C, Liu J, Wang B, Liu X, Wu C, Liu X (2012) Visible photocatalytic degradation of rhodamine B using Fe(III)-substituted phosphotungstic heteropolyanion. J Mol Catalysis A Chem. https://doi.org/10.1016/j.molcata.2012.07.031
Mylonas A, Hiskia A, Androulaki E, Dimotikali D, Papaconstantinou E (1999) New aspect of the mechanism of photocatalytic oxidation of organic compounds by polyoxometalates in aqueous solutions. The selective photooxidation of propan-2-ol to propanone: The role of oh radicals. Phys Chem Chem Phys. https://doi.org/10.1039/a808104b
Walsh JJ, Bond AM, Forster RJ, Keyes TE (2016) Hybrid polyoxometalate materials for photo(electro-) Chemical Applications. Coord Chem Rev. https://doi.org/10.1016/j.ccr.2015.06.016
López X, Bo C, Poblet JM (2002) Electronic properties of polyoxometalates: Electron and Proton affinity of mixed-addenda Keggin and wells−dawson anions. J Am Chem Soc. https://doi.org/10.1021/ja020407z
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16, Revision A.03, Gaussian, Inc., Wallin
Lee C, Yang W, Parr RG (1988) Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys Rev B. https://doi.org/10.1103/physrevb.37.785
Becke AD (1992) Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J Chem Phys. https://doi.org/10.1063/1.462066
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem. https://doi.org/10.1021/j100096a001
Goerigk L, Grimme S (2011) A thorough benchmark of density functional methods for general main group thermochemistry, kinetics, and noncovalent interactions. Phys Chem Chem Phys. https://doi.org/10.1039/c0cp02984j
Sham LJ, Kohn W (1966) One-particle properties of an inhomogeneous interacting electron gas. Phys Rev. https://doi.org/10.1103/physrev.145.561
Salahub DR, Zerner MC (1989) The challenge of D and F electrons: Theory and computation. American Chemical Society, Washington
Parr RG, Yang W (1989) Density-functional theory of atoms and molecules. New York, Oxford University Press, New York
Hay PJ, Wadt WR (1985) ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms SC to hg. J Chem Phys. https://doi.org/10.1063/1.448799
Ochterski JW, Petersson GA, Montgomery JA (1996) A complete basis set model chemistry. V. Extensions to six or more heavy atoms. J Chem Phys. https://doi.org/10.1063/1.470985
Petersson GA, Bennett A, Tensfeldt TG, AlLaham MA, Shirley WA, Mantzaris J (1988) A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J Chem Phys. https://doi.org/10.1063/1.455064
Pascual-ahuir JL, Silla E, Tuñon I (1994) GEPOL: an improved description of molecular surfaces. III. A new algorithm for the computation of a solvent-excluding surface. J Comput Chem. https://doi.org/10.1002/jcc.540151009
Miertuš S, Scrocco E, Tomasi J (1981) Electrostatic interaction of a solute with a continuum. A direct utilizaion of ab initio molecular potentials for the prevision of solvent effects. Chem Phys. https://doi.org/10.1016/0301-0104(81)85090-2
Yang W, Mortier WJ (1986) The use of global and local molecular parameters for the analysis of the gas-phase basicity of Amines. J Am Chem Soc. https://doi.org/10.1021/ja00279a008
Hratchian HP, Schlegel HB (2005) Finding minima, transition states and following reaction pathways on ab initio potential energy surfaces. Theor Appl Comput Chem. https://doi.org/10.1016/b978-044451719-7/50053-6
Fukui K (1981) The path of chemical reactions - the IRC approach. Acc Chem Res. https://doi.org/10.1021/ar00072a001
Jiang N, Schwarz WH, Li J (2015) Theoretical studies on hexanuclear oxometalates [M6L19]q− (m = Cr, Mo, W, Sg, Nd, U). Electronic structures, oxidation states, aromaticity, and stability. Inorg Chem. https://doi.org/10.1021/acs.inorgchem.5b00372
Bannani F, Driss H, Debbabi M, Thouvenot R (2011) A Dimeric Lindqvist-Type Polyoxoanion: New Synthetic Route And Structure Of (nbu4N)4[(TaW5O18)2O]. J La Société Chim Tunisie
Aihara J (1999) Reduced HOMO−LUMO gap as an index of kinetic stability for polycyclic aromatic hydrocarbons. J Phys Chem A. https://doi.org/10.1021/jp990092i
Aihara J (2000) Correlation found between the HOMO–LUMO energy separation and the chemical reactivity at the most reactive site for isolated-pentagon isomers of fullerenes. Phys Chem Chem Phys. https://doi.org/10.1039/b002601h
Guermi IN, Saal A (2023) Theoretical investigation of structural parameters, reactivity behavior, and thermodynamic properties of Anderson polyoxometalate (POM). Struct Chem. https://doi.org/10.1007/s11224-022-02088-7
Wang J, Fu X, Wang J, Hu C (2009) Effects of vanadium (V)-substitution on the oxidative properties of α-keggin-type heteropolyanion clusters—progress in DFT Theoretical Studies. Sci China Series B: Chem. https://doi.org/10.1007/s11426-009-0191-z
Makov G (1995) Chemical hardness in density functional theory. J Phys Chem. https://doi.org/10.1021/j100023a006
Parr RG, Yang W (1984) Density functional approach to the frontier-electron theory of chemical reactivity. J Am Chem Soc. https://doi.org/10.1021/ja00326a036
Maestre JM, Sarasa JP, Bo C, Poblet JM (1998) Ab initio study of the relative basicity of the external oxygen sites in M2W4O194-(m = Nb and V). Inorg Chem. https://doi.org/10.1021/ic960222r
Lan J, Wang Y, Huang B, Xiao Z, Wu P (2021) Application of polyoxometalates in photocatalytic degradation of organic pollutants. Nanoscale Adv. https://doi.org/10.1039/d1na00408e
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to the article and approved the contents of the manuscript. M.N. and M.Z. performed the computational calculations. M.N. performed the interpretations and the first draft of the manuscript. A.S., M.S. and M.Z. edited and reviewed the manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nassar, M., Saal, A., Zhou, M. et al. Insights into substitution effects and reactivity of Lindqvist-type polyoxometalates from DFT calculations. Struct Chem (2023). https://doi.org/10.1007/s11224-023-02236-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11224-023-02236-7