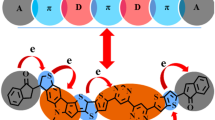
Abstract
The new design of 6,6′-di(thiophen-2-yl)-4,4′-bipyrimidine (DTB)-based organic dyes (TCIT1-TCIT6) for dye-sensitized solar cell (DSSC) application was systematically designed by a donor (D), π-linker, and acceptor (A) design to form the D-π-D-π-A structure. The exchange–correlation (XC) as well as long-range corrected (LC) functionals were utilized to represent these dyes in density functional theory (DFT) and time-dependent DFT (TD-DFT) techniques. The TD-CAM-B3LYP approach was perfectly matched the DTB dye. Energy gaps between highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs) ranged between 1.73-2.92 eV. Their light-harvesting efficiency (LHE) values ranged between 0.001-0.106 eV with dye TCIT5 with the highest value. The open circuit voltage calculated against the DTB as donor moiety ranged 2.45–3.64 eV with positive values to demonstrate their ON position in their solar devices. All the dyes showed a very short excited-state lifetime ranging between 0.0001-0.0051 ns which showed how easily they can shift from an excited- (fluorescent) to ground-state position. Improved results for DSSCs were provided by the intended dyes in addition to their high horizontal dipole moment (µnormal) and open circuit photovoltage (eVOC). It benefits increased efficiency as a result. The findings of the current theoretical analysis show that all D-π-D-π-A dyes may be suitable sensitizers for DSSC applicability.











Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information file.
Code availability
Gaussian 09 W and Gauss view 5.1 are used for simulation, and Origin software is used to draw the plots.
References
Molecular Patching Engineering to Drive Energy Conversion as Efficient and Environment‐Friendly Cell toward Wireless Power Transmission-Shu-2020-Advanced Functional Materials-Wiley Online Library. https://onlinelibrary.wiley.com/doi/abs/10.1002/adfm.201908299. Accessed 9 Apr 2023
Hassan AU, Mohyuddin A, Güleryüz C et al (2022) Novel pull–push organic switches with D–π–A structural designs: computational design of star shape organic materials. Struct Chem. https://doi.org/10.1007/s11224-022-01983-3
Resch-Genger U, Grabolle M, Cavaliere-Jaricot S et al (2008) Quantum dots versus organic dyes as fluorescent labels. Nat Methods 5:763–775
Hassan AU, Sumrra SH, Nazar MF, Güleryüz C (2022) A DFT study on new photovoltaic dyes to investigate their NLO tuning at near infrared region (NIR) as pull–push effect by end capped acceptors. J Fluoresc. https://doi.org/10.1007/s10895-022-03075-1
Richhariya G, Kumar A, Tekasakul P, Gupta B (2017) Natural dyes for dye sensitized solar cell: a review. Renew Sustain Energy Rev 69:705–718. https://doi.org/10.1016/j.rser.2016.11.198
O’Regan B, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740. https://doi.org/10.1038/353737a0
Afolabi SO, Semire B, Idowu MA (2021) Electronic and optical properties’ tuning of phenoxazine-based D-A2-π-A1 organic dyes for dye-sensitized solar cells. DFT/TDDFT investigations. Heliyon 7:e06827. https://doi.org/10.1016/j.heliyon.2021.e06827
Meenakshi R (2017) Spectral investigations, DFT based global reactivity descriptors, Inhibition efficiency and analysis of 5-chloro-2-nitroanisole as $π$-spacer with donor-acceptor variations effect for DSSCs performance. J Mol Struct 1127:694–707
Wang WV, Zhang Y, Li X-Y et al (2021) High performance nonvolatile organic field-effect transistor memory devices based on pyrene diimide derivative. InfoMat 3:814–822. https://doi.org/10.1002/inf2.12186
Hassan AU, Sumrra SH, Mustafa G, Zubair M, Mohyuddin A, Nkungli NK, Imran M (2023) Molecular modeling of mordant black dye for future applications as visible light harvesting materials with anchors: design and excited state dynamics. J Mol Mod 29(74). https://link.springer.com/article/10.1007/s00894-023-05474-y. Accessed 9 Apr 2023
Meena M, Sundararajan RS, Manikandan E, Shalini M (2022) Diffractional, optical, electrical, NLO and surface studies of l-asparagine monohydrate lithium sulphate (LAMLS) single crystals. J Mater Sci: Mater Electron. https://doi.org/10.1007/s10854-022-08734-4
Nosheen E, Shah SM, Hussain H, Murtaza G (2016) Photo-sensitization of ZnS nanoparticles with renowned ruthenium dyes N3, N719 and Z907 for application in solid state dye sensitized solar cells: a comparative study. J Photochem Photobiol, B 162:583–591. https://doi.org/10.1016/j.jphotobiol.2016.07.033
Hassan AU, Sumrra SH, Nkungli NK, Güleryüz C (2022) Theoretical probing of 3d nano metallic clusters as next generation non-linear optical materials. Res Chem 100627. https://doi.org/10.1016/j.rechem.2022.100627
Manfredi N, Cecconi B, Abbotto A (2014) Multi-branched multi-anchoring metal-free dyes for dye-sensitized solar cells. Eur J Org Chem 2014:7069–7086. https://doi.org/10.1002/ejoc.201402422
Sudarsan V (2012) Optical materials: fundamentals and applications. Funct Mater 285–322. https://doi.org/10.1016/B978-0-12-385142-0.00008-8
Tachibana Y, Hara K, Sayama K, Arakawa H (2002) Quantitative analysis of light-harvesting efficiency and electron-transfer yield in ruthenium-dye-sensitized nanocrystalline TiO2 solar cells. Chem Mater 14:2527–2535
Duerto I, Sarasa S, Barrios D et al (2022) Enhancing the temporal stability of DSSCs with novel vinylpyrimidine anchoring and accepting group. Dyes Pigm 203:110310. https://doi.org/10.1016/j.dyepig.2022.110310
Hassan AU, Sumrra SH (2022) Exploration of pull–push effect for novel photovoltaic dyes with A–π–D design: a DFT/TD-DFT investigation. J Fluoresc. https://doi.org/10.1007/s10895-022-03003-3
Noreen S, Sumrra SH (2022) Correlating the charge transfer efficiency of metallic sulfa-isatins to design efficient NLO materials with better drug designs. Biometals. https://doi.org/10.1007/s10534-022-00385-6
Sumrra SH, Arshad Z, Zafar W et al (2022) Metal incorporated aminothiazole-derived compounds: synthesis, density function theory analysis, in vitro antibacterial and antioxidant evaluation. R Soc Open Sci 8:210910. https://doi.org/10.1098/rsos.210910
Hassan AU, Sumrra SH, Mustafa G et al (2023) Creating intense and refined NLO responses by utilizing dual donor structural designs in A-π-D-π-D-π-A type organic switches: computed device parameters. Struct Chem. https://doi.org/10.1007/s11224-023-02138-8
Reynal A, Palomares E (2011) Ruthenium polypyridyl sensitisers in dye solar cells based on mesoporous TiO2. Eur J Inorg Chem 2011:4509–4526. https://doi.org/10.1002/ejic.201100516
Tao J, Vignale G, Tokatly IV (2007) Time-dependent density functional theory: derivation of gradient-corrected dynamical exchange-correlational potentials. Phys Rev B 76:195126. https://doi.org/10.1103/PhysRevB.76.195126
Jacquemin D, Perpète EA, Scalmani G et al (2007) Assessment of the efficiency of long-range corrected functionals for some properties of large compounds. J Chem Phys 126:144105. https://doi.org/10.1063/1.2715573
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57
Ma L-J, Jia J, Wu H-S, Ren Y (2013) Ti–η2-(C2H2) and HCC–TiH as high capacity hydrogen storage media. Int J Hydrogen Energy 38:16185–16192. https://doi.org/10.1016/j.ijhydene.2013.09.151
Rayne S, Forest K (2016) A comparative examination of density functional performance against the ISOL24/11 isomerization energy benchmark. Comput Theor Chem 1090:147–152
Almogati RN, Aziz SG, Hilal R (2017) Effect of substitution on the optoelectronic properties of dyes for DSSC. A DFT approach. J Theor Comput Chem 16:1750018. https://doi.org/10.1142/S0219633617500183
De Angelis F, Fantacci S, Selloni A et al (2010) First-principles modeling of the adsorption geometry and electronic structure of Ru(II) dyes on extended TiO2 substrates for dye-sensitized solar cell applications. J Phys Chem C 114:6054–6061. https://doi.org/10.1021/jp911663k
Zhang J, Li H-B, Sun S-L et al (2012) Density functional theory characterization and design of high-performance diarylamine-fluorene dyes with different π spacers for dye-sensitized solar cells. J Mater Chem 22:568–576. https://doi.org/10.1039/C1JM13028E
Tunç G, Güzel E, Şişman İ et al (2019) Effect of new asymmetrical Zn(II) phthalocyanines on the photovoltaic performance of a dye-sensitized solar cell. New J Chem 43:14390–14401. https://doi.org/10.1039/C9NJ02585E
Hassan AU, Sumrra SH, Zafar W et al (2022) Enriching the compositional tailoring of NLO responsive dyes with diversity oriented electron acceptors as visible light harvesters: a DFT/TD-DFT approach. Mol Phys e2148585. https://doi.org/10.1080/00268976.2022.2148585
Hassan AU, Sumrra SH (2022) Exploring the bioactive sites of new sulfonamide metal chelates for multi-drug resistance: an experimental versus theoretical design. J Inorg Organomet Polym Mater 32:513–535. https://doi.org/10.1007/s10904-021-02135-6
Hassan AU, Mohyuddin A, Nadeem S et al (2022) Structural and electronic (absorption and fluorescence) properties of a stable triplet diphenylcarbene: a DFT study. J Fluoresc. https://doi.org/10.1007/s10895-022-02969-4
Hassan AU, Guleryuz C (2021) Theoretical evaluation of the permeability of the discharge item (LiOOH) in Li-O-2 batteries. Lat Am Appl Res 51:153–157
Hassan AU, Sumrra SH, Zafar MN et al (2022) New organosulfur metallic compounds as potent drugs: synthesis, molecular modeling, spectral, antimicrobial, drug likeness and DFT analysis. Mol Diversity 26:51–72. https://doi.org/10.1007/s11030-020-10157-4
Noreen S, Sumrra SH (2021) Aminothiazole-linked metal chelates: synthesis, density functional theory, and antimicrobial studies with antioxidant correlations. ACS Omega 6:33085–33099
Jackson N, Czaplewski L, Piddock LJV (2018) Discovery and development of new antibacterial drugs: learning from experience? J Antimicrob Chemother 73:1452–1459. https://doi.org/10.1093/jac/dky019
Greisch J-F, Harding ME, Kordel M et al (2013) Intrinsic fluorescence properties of rhodamine cations in gas-phase: triplet lifetimes and dispersed fluorescence spectra. Phys Chem Chem Phys 15:8162–8170. https://doi.org/10.1039/C3CP44362K
Sumrra SH, Hassan AU, Zafar MN et al (2022) Metal incorporated sulfonamides as promising multidrug targets: combined enzyme inhibitory, antimicrobial, antioxidant and theoretical exploration. J Mol Struct 1250:131710. https://doi.org/10.1016/j.molstruc.2021.131710
Vamsikrishna N, Kumar MP, Ramesh G et al (2017) REGULAR ARTICLE DNA interactions and biocidal activity of metal complexes of benzothiazole Schiff bases : synthesis, characterization. J Chem Sci. https://doi.org/10.1007/s12039-017-1273-7
Zhang F, Wu D, Xu Y, Feng X (2011) Thiophene-based conjugated oligomers for organic solar cells. J Mater Chem 21:17590–17600. https://doi.org/10.1039/C1JM12801A
Bhattacharya S, Biswas C, Raavi SSK et al (2019) Synthesis, optical, electrochemical, DFT studies, NLO properties, and ultrafast excited state dynamics of carbazole-induced phthalocyanine derivatives. J Phys Chem C 123:11118–11133
Akin S, Açikgöz S, Gülen M et al (2016) Investigation of the photoinduced electron injection processes for natural dye-sensitized solar cells: the impact of anchoring groups. RSC Adv 6:85125–85134
Guechtouli N, Zaater S, Kichou N et al (2019) Theoretical investigation of dicarboxamide mono copper (II) and novel transition metal complexes: structural, chemical reactivity, vibrational and in-silico biological analysis. J Mol Struct 1188:23–30. https://doi.org/10.1016/j.molstruc.2019.03.068
Aydın G, Koçak O, Güleryüz C, Yavuz I (2020) Structural order and charge transfer in highly strained carbon nanobelts. New J Chem 44:15769–15775. https://doi.org/10.1039/D0NJ03455J
El-Shafiy HF, Shebl M (2019) Binuclear oxovanadium(IV), cerium(III) and dioxouranium(VI) nano complexes of a bis(bidentate) ligand: synthesis, spectroscopic, thermal, DFT calculations and biological studies. J Mol Struct 1194:187–203. https://doi.org/10.1016/j.molstruc.2019.05.063
Hassan AU, Sumrra SH, Imran M, Chohan ZH (2022) New 3d multifunctional metal chelates of sulfonamide: spectral, vibrational, molecular modeling, DFT, medicinal and in silico studies. J Mol Struct 132305. https://doi.org/10.1016/j.molstruc.2021.132305
Dey S (2019) Recent progress in molecular design of fused ring electron acceptors for organic solar cells. Small 15:1900134
Mintmire JW, White CT (1998) Universal density of states for carbon nanotubes. Phys Rev Lett 81:2506
Escudero D, Laurent AD, Jacquemin D (2017) Time-dependent density functional theory: a tool to explore excited states BT - handbook of computational chemistry. In: Kaczmarek-Kedziera A, Puzyn T et al (eds) Leszczynski J. Springer International Publishing, Cham, pp 927–961
Moroz A (1995) Density-of-states calculations and multiple-scattering theory for photons. Phys Rev B 51:2068
Acknowledgements
The authors are grateful to the University of Gujrat, Gujrat, Pakistan, for accessing the all-research facilities. Authors also express their appreciation to the Deanship of Scientific Research at King Khalid University Saudi Arabia for funding through research groups program under grant number R.G.P. 1/92/43.
Author information
Authors and Affiliations
Contributions
Conceptualization: Sajjad H. Sumrra; methodology: Abrar U. Hassan; formal analysis and investigation: Muhammad Imran; writing—original draft preparation: Abrar Mohyuddin; writing—review and editing: Ayesha Mohyuddin; resources: Rana F. Mehmood.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
This article does not have any studies with human participants or animals, clinical trial registration, or plant reproducibility performed by any author.
Consent for publication
All authors have approved the paper and agree with its publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hassan, A.U., Sumrra, S.H., Mustafa, G. et al. Novel pull–push solar switches with a D-π-D-π-A framework of the thiophene core: computed absorbance/fluorescence ability with device parameters. Struct Chem 35, 47–64 (2024). https://doi.org/10.1007/s11224-023-02172-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02172-6