Abstract
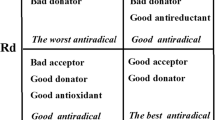
The geometry optimization of a series of twenty-seven lapachol derivatives used for the treatment of carcinosarcoma tumor (Walker 256) is performed at B3LYP/6–311++G(d,p) level in three distinct media (gas phase, water, and acetonitrile). The migration from the E to the Z configuration or vice versa provokes a noticeable change in the O–H bond distance and potentially a remarkable alteration of the antioxidant activity. In addition, the majority of the E configuration structures have shown a more pronounced antioxidant activity in solution (water and acetonitrile). In their various Z/E configurations, the 2-hydroxy-3-(2,3-dihydroxy-3-methylbutyl)-1,4-naphtoquinone compound bearing O···HO hydrogen bond (La21(HB)) as well as the (2-hydroxy-3-(but-2-enyl)-1,4-naphtoquinone (La6(HB)) and 2-hydroxy-3-(3-methylpent-2-enyl)-1,4-naphtoquinone (La7(HB)) compounds have stronger antioxidant powers than classical bioactive compounds (caffeic acid, ferulic acid, p-coumaric acid, sinapic acid, and vitamin C). On the whole, however, all Z configuration compounds have a higher antiradical status compared to that of their E configuration counterparts. From the global donor–acceptor mappings, the 2-hydroxy-3-(2,3-dihydroxy-3-methylbutyl)-1,4-naphtoquinone structure (La21 (HB)) has been elected as the best antiradical molecule of the series in the three study media. Its antiradical power has been detected to be higher than that of these five standard references adopted.






Similar content being viewed by others
Availability of data and material
The data used to support the finding of this study are available.
References
Mammino L (2012) Computational chemistry capacity building in an underprivileged context: challenges, outcomes and perspectives. Tanz J Sc 38(3):95–107
Ponnan A, Perumal R, Sathiyavedu TS, Arabandi R (2006) Antioxidant activity measured in different solvent fractions obtained from Mentha spicata Linn. An analysis by ABTS decolorization assay. Asia Pac J Clin Nutr 119–124
Zhulong C, Chun-Peng S, Woe YK, Ken Y (2016) ROS regulation during plant abiotic stress responses. Front Plant Sci 1–3
Kris-Etherton P, Le Fevre M, Beecher G, Gross M, Keen C, Etherton T (2004) Bioactive compounds in nutrition and health-research methodologies for establishing biological function: the antioxidant and anti-inflammatory effects of flavonoids on atherosclerosis. Annu Rev Nutr 24:511–538
Schwarz KG, Bertelsen LR, Nissen PT, Gardner MI, Heinonen A, Hopia T, HuynhBa P, Lambelet D, McPhall LH, Skibsted HL, Tijburg L (2001) Investigation of plant extracts for the protection of processed foods against lipid oxidation. Comparison of antioxidant assays based on radical scavenging, lipid oxidation and analysis of the principal antioxidant compounds. Eur Food Res Technol 212:319–328
Ahn DU, Olson DG, Lee JI, Jo C, Wu C, Chen X (1998) Packaging and irradiation effects on lipid oxidation and volatiles in pork patties. J Food Sci 63:15–17
Shahid W, Ahmad A, Mangaiyarkarasi R, Omer M, Shahina N, Abdurraheem U, Rahmanullah S, Za Y (2013) Effect of polyphenolic rich, green tea extract as antioxidant on broiler performance during 0–4. IJAR 1(9):177–181
Asare GA, Mossanda KS, Kew MC, Paterson AC, Kahler-Venter CP, Siziba K (2006) Hepatocellular carcinoma caused by iron overload: a possible mechanism of direct hepatocarcinogenicity. Toxicology 219:41–52
Haber F, Weiss J (1934) The catalytic decomposition of hydrogen peroxide by iron salts. Proc R Soc Lond A Math Phys Sci 147:332–335
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Shahidi F, Zhong Y (2015) Measurement of antioxidant activity. J Funct Foods 8:757–781
Gonzalez-Paramás AM, Esteban S, Santos-Buelga C, de Pascual-Teresa S, Rivas-Gonzalo JC (2004) Flavanol content and antioxidant activity in winery byproducts. J Agric Food Chem 52:234–238
Yilmaz Y, Toledo RT (2004) Major flavonoids in grape seeds and skins: antioxidant capacity of catechin, epicatechin, and gallic acid. J Agric Food Chem 52:255–260
Ruberto G, Renda A, Daquino C, Amico V, Spatafora C, Tringali C, Tommasi N (2007) Polyphenols constituents and antioxidant activity of grape pomace from five Sicilian red grape cultivars. Food Chem 100:203–210
Newkirk KA, Gilchrist L, Hand LW, Sutton DS (1993) Effects of synthetic antioxidants and rosemary extracts on oxidative rancidity and color stability in whole hog sausage. Animal Science Research Report. Oklahoma Agricultural Experiment Station 78–83
Nahm SH, Juliana RH, Simon EJ (2012) Effects of selected synthetic and natural antioxidants on the oxidative stability of shea butter (Vitellariaparadoxa subsp. paradoxa). J Med Act Plants 1(2):69–75
Damisi JA, Hansen RW, Grabowsk HG (2003) The price of innovation: new estimates of drugs development cost. J Health Econ 22:185
Chebude Y, Clough J, Crawford B, Engida T, Lancaster S, Marriot R, Midiwo J, Mohee R, Nyamori V, Palermo A, Ruthven S, Welton T (2011) Wealth not waste: green science and engineering for sustainable growth in Africa, Pan African Chemistry Network. RSC Adv 1–21
Krystona TB, Georgieva AB, Pissis P, Georgakilasa AG (2011) Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res Fundam Mol Mech Mutagen 711:193–201
Jiranusornkul S, Laughton CA (2008) Destabilization of DNA duplexes by oxidative damage at guanine: implications for lesion recognition and repair. J R Soc Interface 5:191–198
Konsta AA, Visvardis EE, Haveles KS, Georgakilas AG, Sideris EG (2003) Detecting radiation-induced DNA damage: from changes in dielectric properties to programmed cell death. J Non-Cryst Solids 305:295–302
Spassky A, Angelov D (1997) Influence of the local helical conformation on the guanine modifications generated from one-electron DNA oxidation. Biochemistry 36:6571–6576
Gillard N, Begusova M, Castaing B, Potheim-Maurizot M (2004) Radiation effects binding of Fgp repair protein to an abasic site containing DNA. Radiat Res 162:566–571
Boveris A (1982) Superoxide radical and hydrogen peroxide in mitochondria, ed. W.A. Pryor. Academic Press, New York. Free Radic Biol 65–89
Ionescu JG, Merk M, Dowes F (1998) Clinical application of redox potential testing in the blood. Syllabus of 33rd AAEM Annual Meeting, Baltimore, USA, pp 503–521
Seo MY, Lee SM (2002) Protective effect of low dose of ascorbic acid on hepabiliary function in hepatic ischemia/reperfusion in rats. J Hepatol 36(1):72–77
Duarte TL, Lunec J (2005) When is an antioxidant not an antioxidant? A review of novel actions and reactions of vitamin C. Free Radic Res 39:671–681
Blumberg J, Block G (1994) The alpha-tocopherol beta-carotene cancer prevention in Finland. Nutr Rev 52(7):242–245
Misra HP, Fridovich I (1972) The role of superoxide anion in the auto-oxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Bolton JL, Trush MA, Penning G, Dryhurst G, Monks TJ (2000) Role of quinones in toxicology. 98. Chem Res Toxicol 13:135–160
Boveris A, Docampo R, Turrens JF, Stoppani AO (1978) Effect of B-lapachone on superoxide anion and hydrogen peroxide production in Trypanosoma cruzi. Biochem J 175:431–439
Hobb C (1998) Herbal remedies for dummies. John Wiley & Sons Ltd, Chichester
EFSA (2012) Scientific opinion on the reevaluation of butylated hydroxytoluene BHT (E 321) as a Food Additive. EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). EFSA J 10(3):2588
Subramanian S, Ferreira MC, Trsic M (1998) A structure-activity relationship study of lapachol and some derivatives of 1, 4-naphthoquinones against carcinosarcoma Walker 256. Struct Chem 9(1):47–57
Kabanda MM, Mammino L, Murulana LC, Mwangi HM, Mabusela WT (2015) Antioxidant radical scavenging properties of phenolic pent-4-en-1-yne derivatives isolated from Hypoxis rooper. A DFT Study in vacuo and in Solution. Int J Food Prop 18:149–164
Leopoldini M, Pitarch IP, Russo N, Toscano M (2004) Structure, conformation, and electronic properties of apigenin, luteolin, and taxifolin antioxidants. A first principle theoretical study. J Phys Chem A 108(92–96):23
Leopoldini M, Marino T, Russo N, Toscano M (2004) Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J Phys Chem A 108:4916–4922
Leopoldini M, Marino T, Russo N, Toscano M (2004) Density functional computations of the energetic and spectroscopic parameters of quercetin and its radicals in the gas phase and in solvent. Theor Chem Acc 111:210–216
Zhang G, Musgrave CB (2007) Comparison of DFT methods for molecular orbital eigenvalue calculations. J Phys Chem A 111:1554–1561
Delgado Alfaro RA, Gomez-Sandoval Z, Mammino L (2014) Evaluation of the antiradical activity of hyperjovinol-A utilizing donor-acceptor map. J Mol Model 20:2337
Gordon MS, Freitag MA, Bandyopadhyay P, Jensen JH, Kairys V, Stevens WJ (2001) The effective fragment potential method: a QMbased MM approach to modeling environmental effects in chemistry. J Phys Chem A 105(293307):20
Cao GH, Prior RL (1999) Measurement of oxygen radical absorbance capacity in biological samples. Meth Enzymol 299:50–62
Lien E, Ren S, Bui H, Wang R (1999) Quantitative structure activity relationship analysis of phenolic antioxidants. Free Radic Biol Med 26(3–4):285–294
Martίnez A (2009) Donator acceptor map of psittacofulvins and anthocyanins: are they good antioxidant substances? J Phys Chem B 113:4915–4921
Johns JR, James A (2014) Platts, Theoretical insight into the antioxidant properties of melatonin and derivatives. Org Biomol Chem 12:7820–7827
Geerling P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103:1793–1873
Gàzques JL, Cedillo A, Vela A (2007) Electrodonating and electroaccepting. J Phys Chem A 111:1966–1970
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JRMontgomery Jr JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi JJ, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth A, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2009) Gaussian 09: revision A.2. Gaussian, Inc.: Wallingford, CT
Fifen JJ, Nsangou M, Dhaouadi Z, Motapon O, Jaidane N (2011) Solvent effects on the antioxidant activity of 3,4-dihydroxyphenylpyruvic acid : DFT and TD-DFT studies. Comput Theor Chem 966:232–243
Menzel H (2002) Photoreactive organic thin films. Zouheir Sekkat-Wolfgang Knoll: Elsevier Science (USA)
Parr RG, Szentpály LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924
Parr RG, Chattaraj PK (1991) Principle of maximum hardness. J Am Chem Soc 113:1854–1855
Chamorro E, Chattaraj P, Fuentealba KP (2003) Variation of the electrophilicity index along the reaction path. J Phys Chem A 107:7068–7072
Salga MS, Sada I, Abdullahi M (2014) Influence of steric hindrance on the antioxidant activity of some Schiff base ligands and their copper (II) complexes. Orient J Chem 30(4):1529–1534
Lengyel J, Rimarčík J, Vagànek A, Klein E (2013) On the radical scavenging activity of isoflavones: thermodynamics of O-H bond cleavage. Phys Chem Chem Phys 15:10895–10903
Manach C, Scalbert A, Morand C, Remesy C, Jimenez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
Wright JS, Johnson ER, DiLabio GA (2001) Predicting the antioxidant activity of phenolic antioxidant: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc 123:1173–1183
Javan AJ, Javan MJ, Tehrani AA (2013) Theoretical investigation on antioxidant activity of bromophenol from the marine red alga Rhodomela confervoides: H-atom versus electron transfer mechanism. J Agric Food Chem 61:1534–1541
Mezsam N (2014) On the antioxidant activity of the ortho and meta substituted daidzein derivatives in the gas phase and solvent environment. J Mex Chem Soc 58:36–45
Zang HY (2005) Structure-activity relationships and rational design strategies for radical-scavenging antioxidants. Curr Comput-Aided Drug Des 1:257–273
Mbieda JN, Ateba AB, Bikele MD, ZoboHoltomo MJO, Toze FA (2020) Insight into the antioxidant and antiradical properties of coloratane sesquiterpenes extracted from warburgia ugandensis: theoretical evaluation. Struc Chem 020:01634–01645
Acknowledgements
We express our sincere gratitude to Dr. Moto Ongagna Jean and Dr. Adjieufak Abel Idrice of the University of Douala for the facilities provided for geometry optimization.
Funding
Financial support from the Ministry of Higher Education of Cameroon.
Author information
Authors and Affiliations
Contributions
For the elaboration of this work, the tasks are divided as follows: optimization of geometry and other computational calculations, Djafarou Ngouh Pajoudoro and Inocent Djacktayang; data analysis, Tables 1, 2, and 3 setup, Figs. 1, 2, 3, 4, 5, and 6, additional materials, Tables 1S and 2S and Fig. 1S, Flavien Aristide A Toze and Daniel Lissouck; and article design, coordination, and manuscript writing, Désiré Bikele Mama.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pajoudoro, D.N., Djacktayang, I., Toze, F.A.A. et al. Investigation of the influence of Z/E configuration on the antioxidant and antiradical activities of lapachol and its derivatives: DFT assessment. Struct Chem 34, 979–993 (2023). https://doi.org/10.1007/s11224-022-02061-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-02061-4