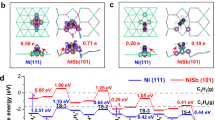
Abstract
Nanocluster models were investigated to explore the diversity of metallacycle intermediates for ethylene dimerization over NiMCM-41 at B3LYP/6-311+G* and M06/Def2-TZVP. The thermodynamic favorability of the formation of matallacycle with respect to the ring size of silica varied in the sequence of 6T < 3T < 2T < 5T < 4T in terms of Gibbs free energy (ranging from − 10.01 to 16.66 kcal/mol at B3LYP/6-311+G*). The reaction cycle faced lower barriers on 3T and 2T clusters, however. The formation of the intermediate and π complexation of 1-butene led to positive total charges on the hydrocarbon segment of the complex, being maximized on four-membered sites and minimized on two-membered ones. Further insights are also provided with QTAIM, frontier orbital, and FTIR analyses.





Similar content being viewed by others
References
Ghashghaee M, Shirvani S (2018) Two-step thermal cracking of an extra-heavy fuel oil: experimental evaluation, characterization, and kinetics. Ind Eng Chem Res 57(22):7421–7430. https://doi.org/10.1021/acs.iecr.8b00819
Karimzadeh R, Godini HR, Ghashghaee M (2009) Flowsheeting of steam cracking furnaces. Chem Eng Res Des 87:36–46. https://doi.org/10.1016/j.cherd.2008.07.009
Karimzadeh R, Ghashghaee M, Nouri M (2010) Effect of solvent dearomatization and operating conditions in steam pyrolysis of a heavy feedstock. Energy Fuel 24(3):1899–1907
Karimzadeh R, Ghashghaee M (2008) Design of a flexible pilot plant reactor for the steam cracking process. Chem Eng Technol 31(2):278–286. https://doi.org/10.1002/ceat.200700326
Ghashghaee M, Karimzadeh R (2011) Multivariable optimization of thermal cracking severity. Chem Eng Res Des 89(7):1067–1077. https://doi.org/10.1016/j.cherd.2010.12.002
Ghashghaee M, Karimzadeh R (2007) Dynamic modeling and simulation of steam cracking furnaces. Chem Eng Technol 30(7):835–843. https://doi.org/10.1002/ceat.200700028
Shirvani S, Ghashghaee M (2018) Combined effect of nanoporous diluent and steam on catalytic upgrading of fuel oil to olefins and fuels over USY catalyst. Pet Sci Technol 36(11):750–755. https://doi.org/10.1080/10916466.2018.1445104
Jafari Fesharaki M, Ghashghaee M, Karimzadeh R (2013) Comparison of four nanoporous catalysts in thermocatalytic upgrading of vacuum residue. J Anal Appl Pyrolysis 102:97–102
Ghashghaee M, Shirvani S, Ghambarian M, Eidi A (2018) Two-stage thermocatalytic upgrading of fuel oil to olefins and fuels over a nanoporous hierarchical acidic catalyst. Pet Sci Technol. https://doi.org/10.1080/10916466.10912018.11463257
Hajheidary M, Ghashghaee M, Karimzadeh R (2013) Olefins production from LPG via dehydrogenative cracking over three ZSM-5 catalysts. J Sci Ind Res 72(12):760–766
Ghashghaee M, Karimzadeh R (2013) Applicability of protolytic mechanism to steady-state heterogeneous dehydrogenation of ethane revisited. Microporous Mesoporous Mater 170:318–330. https://doi.org/10.1016/j.micromeso.2012.12.005
Breuil P-AR, Magna L, Olivier-Bourbigou H (2015) Role of homogeneous catalysis in oligomerization of olefins: focus on selected examples based on group 4 to group 10 transition metal complexes. Catal Lett 145(1):173–192. https://doi.org/10.1007/s10562-014-1451-x
Ghashghaee M (2018) Heterogeneous catalysts for gas-phase conversion of ethylene to higher olefins. Rev Chem Eng 34(5):595–655. https://doi.org/10.1515/revce-2017-0003
Balcar H, Čejka J (2013) Mesoporous molecular sieves as advanced supports for olefin metathesis catalysts. Coord Chem Rev 257(21–22):3107–3124. https://doi.org/10.1016/j.ccr.2013.07.026
Martínez A, Arribas MA, Concepción P, Moussa S (2013) New bifunctional Ni–H-Beta catalysts for the heterogeneous oligomerization of ethylene. Appl Catal A Gen 467:509–518. https://doi.org/10.1016/j.apcata.2013.08.021
Ghashghaee M, Farzaneh V (2018) Nanostructured hydrotalcite-supported RuBaK catalyst for direct conversion of ethylene to propylene. Russ J Appl Chem 91(6):970–974. https://doi.org/10.1134/S1070427218060149
Andrei RD, Popa MI, Fajula F, Hulea V (2015) Heterogeneous oligomerization of ethylene over highly active and stable Ni-AlSBA-15 mesoporous catalysts. J Catal 323:76–84. https://doi.org/10.1016/j.jcat.2014.12.027
Andrei RD, Mureseanu M, Popa MI, Cammarano C, Fajula F, Hulea V (2015) Ni-exchanged AlSBA-15 mesoporous materials as outstanding catalysts for ethylene oligomerization. Eur Phys J Spec Top 224(9):1831–1841. https://doi.org/10.1140/epjst/e2015-02502-0
Zhang H, Li X, Zhang Y, Lin S, Li G, Chen L, Fang Y, Xin H, Li X (2014) Ethylene oligomerization over heterogeneous catalysts. Energ Environ Focus 3(3):246–256. https://doi.org/10.1166/eef.2014.1107
Tanaka M, Itadani A, Kuroda Y, Iwamoto M (2012) Effect of pore size and nickel content of Ni-MCM-41 on catalytic activity for ethene dimerization and local structures of nickel ions. J Phys Chem C 116(9):5664–5672. https://doi.org/10.1021/jp2103066
Iwamoto M (2011) One step formation of propene from ethene or ethanol through metathesis on nickel ion-loaded silica. Molecules 16(9):7844–7863
Choo H, Kevan L (2001) Catalytic study of ethylene dimerization on Ni(II)-exchanged clinoptilolite. J Phys Chem B 105(27):6353–6360. https://doi.org/10.1021/jp0106909
Nkosi B, Ng FTT, Rempel GL (1997) The oligomerization of butenes with partially alkali exchanged NiNaY zeolite catalysts. Appl Catal A Gen 158(1):225–241. https://doi.org/10.1016/S0926-860X(96)00420-6
Sohn JR, Park JH (2001) Characterization of dealuminated NiY zeolite and effect of dealumination on catalytic activity for ethylene dimerization. Appl Catal A Gen 218(1–2):229–234. https://doi.org/10.1016/S0926-860X(01)00649-4
Ng FTT, Creaser DC (1994) Ethylene dimerization over modified nickel exchanged Y-zeolite. Appl Catal A Gen 119(2):327–339. https://doi.org/10.1016/0926-860X(94)85200-6
Ye J, Gagliardi L, Cramer CJ, Truhlar DG (2017) Single Ni atoms and Ni4 clusters have similar catalytic activity for ethylene dimerization. J Catal 354(Supplement C):278–286. https://doi.org/10.1016/j.jcat.2017.08.011
Finiels A, Fajula F, Hulea V (2014) Nickel-based solid catalysts for ethylene oligomerization - a review. Catal Sci Technol 4(8):2412–2426. https://doi.org/10.1039/c4cy00305e
Hulea V, Fajula F (2004) Ni-exchanged AlMCM-41—an efficient bifunctional catalyst for ethylene oligomerization. J Catal 225(1):213–222. https://doi.org/10.1016/j.jcat.2004.04.018
Martínez A, Arribas MA, Moussa S (2015) Development of bifunctional Ni-based catalysts for the heterogeneous oligomerization of ethylene to liquids. In: Kanellopoulos N (ed) Small-scale gas to liquid fuel synthesis. CRC Press, Boca Raton, pp 377–400. https://doi.org/10.1201/b18075-14
Frey AS, Hinrichsen O (2012) Comparison of differently synthesized Ni(Al)MCM-48 catalysts in the ethene to propene reaction. Microporous Mesoporous Mater 164:164–171. https://doi.org/10.1016/j.micromeso.2012.07.015
Alvarado Perea L, Wolff T, Veit P, Hilfert L, Edelmann FT, Hamel C, Seidel-Morgenstern A (2013) Alumino-mesostructured Ni catalysts for the direct conversion of ethene to propene. J Catal 305:154–168. https://doi.org/10.1016/j.jcat.2013.05.007
Alvarado Perea L, Wolff T, Hamel C, Seidel-Morgenstern A (2014) Direct conversion of ethene to propene on Ni/AlMCM-41–study of the reaction mechanism. Jahrestreffen Deutscher Katalytiker, Weimar
Alvarado Perea L (2014) Direct conversion of ethene to propene on Ni-alumino-mesostructured catalysts: synthesis, characterization and catalytic testing. PhD, Otto-von-Guericke Universität, Magdeburg
Iwamoto M, Kosugi Y (2007) Highly selective conversion of ethene to propene and butenes on nickel ion-loaded mesoporous silica catalysts. J Phys Chem C 111(1):13–15
Taoufik M, Le Roux E, Thivolle-Cazat J, Basset J-M (2007) Direct transformation of ethylene into propylene catalyzed by a tungsten hydride supported on alumina: trifunctional single-site catalysis. Angew Chem 119:7340–7343
Taoufik M, Le Roux E, Thivolle-Cazat J, Basset J-M (2007) Direct transformation of ethylene into propylene catalyzed by a tungsten hydride supported on alumina: trifunctional single-site catalysis. Angew Chem Int Ed 46:7202–7205
Iwamoto M (2008) Conversion of ethene to propene on nickel ion-loaded mesoporous silica prepared by the template ion exchange method. Catal Surv Jpn 12(1):28–37. https://doi.org/10.1007/s10563-007-9036-y
Lehmann T, Wolff T, Zahn VM, Veit P, Hamel C, Seidel-Morgenstern A (2011) Preparation of Ni-MCM-41 by equilibrium adsorption—catalytic evaluation for the direct conversion of ethene to propene. Catal Commun 12(5):368–374. https://doi.org/10.1016/j.catcom.2010.10.018
Zahn VM, Wolff T, Lehmann T, Hamel C, Seidel-Morgenstern A (2010) Direct synthesis of propene using supported bifunctional nickel catalysts: preparation and potential. In: ISCRE 21-21st International Symposium on Chemical Reaction Engineering
Lehmann T, Seidel-Morgenstern A (2012) Comment on “Effect of pore size and nickel content of Ni-MCM-41 on catalytic activity for ethene dimerization and local structures of nickel ions”. J Phys Chem C 116(42):22646–22648. https://doi.org/10.1021/jp303935b
Ikeda K, Kawamura Y, Yamamoto T, Iwamoto M (2008) Effectiveness of the template-ion exchange method for appearance of catalytic activity of Ni–MCM-41 for the ethene to propene reaction. Catal Commun 9(1):106–110. https://doi.org/10.1016/j.catcom.2007.05.032
Andrei RD, Popa MI, Fajula F, Cammarano C, Khudhair AA, Bouchmella K, Mutin PH, Hulea V (2015) Ethylene to propylene by one-pot catalytic cascade reactions. ACS Catal 5(5):2774–2777. https://doi.org/10.1021/acscatal.5b00383
Cai FX, Lepetit C, Kermarec M, Olivier D (1987) Dimerization of ethylene into 1-butene over supported tailor-made nickel catalysts. J Mol Catal 43(1):93–116. https://doi.org/10.1016/0304-5102(87)87024-4
Bonneviot L, Olivier D, Che M (1983) Dimerization of olefins with nickel-surface complexes in X-type zeolite or on silica. J Mol Catal 21(1):415–430. https://doi.org/10.1016/0304-5102(93)80138-K
McGuinness DS (2011) Olefin oligomerization via metallacycles: dimerization, trimerization, tetramerization, and beyond. Chem Rev 111(3):2321–2341. https://doi.org/10.1021/cr100217q
Speiser F, Braunstein P, Saussine L (2005) Catalytic ethylene dimerization and oligomerization: recent developments with nickel complexes containing P, N-chelating ligands. Acc Chem Res 38(10):784–793
Brogaard RY, Olsbye U (2016) Ethene oligomerization in Ni-containing zeolites: theoretical discrimination of reaction mechanisms. ACS Catal 6(2):1205–1214. https://doi.org/10.1021/acscatal.5b01957
Šponer JE, Sobalík Z, Leszczynski J, Wichterlová B (2001) Effect of metal coordination on the charge distribution over the cation binding sites of zeolites. A combined experimental and theoretical study. J Phys Chem B 105(35):8285–8290. https://doi.org/10.1021/jp010098j
Rice MJ, Chakraborty AK, Bell AT (2000) Site availability and competitive siting of divalent metal cations in ZSM-5. J Catal 194(2):278–285. https://doi.org/10.1006/jcat.2000.2977
Rice MJ, Chakraborty AK, Bell AT (2000) Theoretical studies of the coordination and stability of divalent cations in ZSM-5. J Phys Chem B 104(43):9987–9992. https://doi.org/10.1021/jp0009352
Neiman ML (2002) Interaction of cobalt and nickel with the hydroxylated silanol silica surface: a theoretical study. MSc, Lehigh University
Balar M, Azizi Z, Ghashghaee M (2016) Theoretical identification of structural heterogeneities of divalent nickel active sites in NiMCM-41 nanoporous catalysts. J Nanostruct Chem 6(4):365–372. https://doi.org/10.1007/s40097-016-0208-z
Ghashghaee M, Shirvani S, Ghambarian M (2017) Kinetic models for hydroconversion of furfural over the ecofriendly Cu-MgO catalyst: an experimental and theoretical study. Appl Catal A Gen 545:134–147. https://doi.org/10.1016/j.apcata.2017.07.040
Ghambarian M, Azizi Z, Ghashghaee M (2017) Cluster modeling and coordination structures of Cu+ ions in Al-incorporated Cu-MEL catalysts—a density functional theory study. J Mex Chem Soc 61(1):1–13. https://doi.org/10.29356/jmcs.v61i1.122
Pidko EA, van Santen RA (2007) Activation of light alkanes over zinc species stabilized in ZSM-5 zeolite: a comprehensive DFT study. J Phys Chem C 111(6):2643–2655. https://doi.org/10.1021/jp065911v
Dědeček J, Sklenak S, Li C, Wichterlová B, Gábová V, Brus J, Sierka M, Sauer J (2009) Effect of Al−Si−Al and Al−Si−Si−Al pairs in the ZSM-5 zeolite framework on the 27Al NMR spectra. A combined high-resolution 27Al NMR and DFT/MM study. J Phys Chem C 113(4):1447–1458. https://doi.org/10.1021/jp8068333
Lesthaeghe D, Van der Mynsbrugge J, Vandichel M, Waroquier M, Van Speybroeck V (2011) Full theoretical cycle for both ethene and propene formation during methanol-to-olefin conversion in H-ZSM-5. ChemCatChem 3(1):208–212. https://doi.org/10.1002/cctc.201000286
Zhang B, Lu Y, He H, Wang J, Zhang C, Yu Y, Xue L (2011) Experimental and density functional theory study of the adsorption of N2O on ion-exchanged ZSM-5: part II. The adsorption of N2O on main-group ion-exchanged ZSM-5. J Environ Sci 23(4):681–686. https://doi.org/10.1016/S1001-0742(10)60482-2
Ghashghaee M, Ghambarian M, Azizi Z (2016) Characterization of extra framework Zn2+ cationic sites in silicalite-2: a computational study. Struct Chem 27(2):467–475. https://doi.org/10.1007/s11224-015-0575-y
Ghambarian M, Ghashghaee M, Azizi Z (2017) Coordination and siting of Cu+ ion adsorbed into silicalite-2 porous structure: a density functional theory study. Phys Chem Res 5(1):135–152. https://doi.org/10.22036/pcr.2017.39255
Ghambarian M, Azizi Z, Ghashghaee M (2016) Diversity of monomeric dioxo chromium species in Cr/silicalite-2 catalysts: a hybrid density functional study. Comput Mater Sci 118:147–154. https://doi.org/10.1016/j.commatsci.2016.03.009
Becke AD (1988) Density-functional exchange-energy approximation with correct asymptotic behavior. Phys Rev A 38(6):3098–3100. https://doi.org/10.1103/PhysRevA.38.3098
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98(7):5648–5652. https://doi.org/10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37(2):785–789. https://doi.org/10.1103/PhysRevB.37.785
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Accounts 120(1–3):215–241. https://doi.org/10.1007/s00214-007-0310-x
Hariharan PC, Pople JA (1974) Accuracy of AHn equilibrium geometries by single determinant molecular orbital theory. Mol Phys 27(1):209–214. https://doi.org/10.1080/00268977400100171
Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pople JA (1982) Self-consistent molecular orbital methods. XXIII. A polarization-type basis set for second-row elements. J Chem Phys 77(7):3654–3665. https://doi.org/10.1063/1.444267
Clark T, Chandrasekhar J, Spitznagel GW, Schleyer PVR (1983) Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li–F. J Comput Chem 4(3):294–301. https://doi.org/10.1002/jcc.540040303
Frisch MJ, Pople JA, Binkley JS (1984) Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J Chem Phys 80(7):3265–3269. https://doi.org/10.1063/1.447079
Weigend F (2006) Accurate Coulomb-fitting basis sets for H to Rn. Phys Chem Chem Phys 8(9):1057–1065. https://doi.org/10.1039/b515623h
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7(18):3297–3305. https://doi.org/10.1039/b508541a
Feller D (1996) The role of databases in support of computational chemistry calculations. J Comput Chem 17(13):1571–1586. https://doi.org/10.1002/(sici)1096-987x(199610)17:13<1571::aid-jcc9>3.0.co;2-p
Ferullo RM, Garda GR, Belelli PG, Branda MM, Castellani NJ (2006) Deposition of small Cu, Ag and Au particles on reduced SiO2. J Mol Struct 769(1–3):217–223. https://doi.org/10.1016/j.theochem.2006.03.048
Göltl F, Hafner J (2012) Structure and properties of metal-exchanged zeolites studied using gradient-corrected and hybrid functionals. III. Energetics and vibrational spectroscopy of adsorbates. J Chem Phys 136(6):064503-064501–064503-064531. https://doi.org/10.1063/1.3676410
Chen K, Wang Z-C, Schlangen M, Wu Y-D, Zhang X, Schwarz H (2011) Thermal activation of methane and ethene by bare MO.+ (M=Ge, Sn, and Pb): a combined theoretical/experimental study. Chem Eur J 17(35):9619–9625. https://doi.org/10.1002/chem.201101538
Ghashghaee M, Ghambarian M (2018) Methane adsorption and hydrogen atom abstraction at diatomic radical cation metal oxo clusters: first-principles calculations. Mol Simul 44(10):850–863. https://doi.org/10.1080/08927022.2018.1465568
Firouzbakht M, Zhou S, González-Navarrete P, Schlangen M, Kaupp M, Schwarz H (2017) Metal-dependent strengthening and weakening of M−H and M−C bonds by an oxo ligand: thermal gas-phase activation of methane by [OMH]+ and [MH]+ (M=Mo, Ti). Chem Eur J. https://doi.org/10.1002/chem.201701615
Mardirossian N, Head-Gordon M (2017) Thirty years of density functional theory in computational chemistry: an overview and extensive assessment of 200 density functionals. Mol Phys 115(19):2315–2372. https://doi.org/10.1080/00268976.2017.1333644
Kendall RA, Dunning TH, Harrison RJ (1992) Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J Chem Phys 96(9):6796–6806. https://doi.org/10.1063/1.462569
Glendening E, Badenhoop J, Reed A, Carpenter J, Weinhold F (1996) NBO 3.1. Theoretical Chemistry Institute, University of Wisconsin, Madison
Rodríguez JI, Bader RFW, Ayers PW, Michel C, Götz AW, Bo C (2009) A high performance grid-based algorithm for computing QTAIM properties. Chem Phys Lett 472(1–3):149–152. https://doi.org/10.1016/j.cplett.2009.02.081
Bader RFW (1991) A quantum theory of molecular structure and its applications. Chem Rev 91(5):893–928. https://doi.org/10.1021/cr00005a013
Bader RFW (2005) The quantum mechanical basis of conceptual chemistry. Monatsh Chem 136(6):819–854. https://doi.org/10.1007/s00706-005-0307-x
Bader RFW (1975) Molecular fragments or chemical bonds. Acc Chem Res 8(1):34–40. https://doi.org/10.1021/ar50085a005
Bader RFW (1994) Atoms in molecules: a quantum theory, vol 22. International Series of Monographs on Chemistry. Oxford University Press, Oxford
Matta CF, Boyd RJ (2007) The quantum theory of atoms in molecules: from solid state to DNA and drug design. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Valiev M, Bylaska EJ, Govind N, Kowalski K, Straatsma TP, Van Dam HJJ, Wang D, Nieplocha J, Apra E, Windus TL, de Jong WA (2010) NWChem: a comprehensive and scalable open-source solution for large scale molecular simulations. Comput Phys Commun 181(9):1477–1489. https://doi.org/10.1016/j.cpc.2010.04.018
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592. https://doi.org/10.1002/jcc.22885
Bruno IJ, Cole JC, Edgington PR, Kessler M, Macrae CF, McCabe P, Pearson J, Taylor R (2002) New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr B 58(3 Part 1):389–397. https://doi.org/10.1107/S0108768102003324
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J, Wood PA (2008) Mercury CSD 2.0—new features for the visualization and investigation of crystal structures. J Appl Crystallogr 41(2):466–470. https://doi.org/10.1107/S0021889807067908
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, van de Streek J (2006) Mercury: visualization and analysis of crystal structures. J Appl Crystallogr 39(3):453–457. https://doi.org/10.1107/S002188980600731X
Taylor R, Macrae CF (2001) Rules governing the crystal packing of mono- and dialcohols. Acta Crystallogr B 57(6):815–827. https://doi.org/10.1107/S010876810101360X
Van Hemelrijk D, Van den Enden L, Geise HJ, Sellers HL, Schaefer L (1980) Structure determination of 1-butene by gas electron diffraction, microwave spectroscopy, molecular mechanics, and molecular orbital constrained electron diffraction. J Am Chem Soc 102(7):2189–2195. https://doi.org/10.1021/ja00527a007
Sierraalta A, Añez R, Brussin M-R (2002) Theoretical study of NO2 adsorption on a transition-metal zeolite model. J Catal 205(1):107–114. https://doi.org/10.1006/jcat.2001.3425
Gatti C (2005) Chemical bonding in crystals: new directions. Z Krist 220:399–457. https://doi.org/10.1524/zkri.220.5.399.65073
Alecu IM, Zheng J, Zhao Y, Truhlar DG (2010) Computational thermochemistry: scale factor databases and scale factors for vibrational frequencies obtained from electronic model chemistries. J Chem Theory Comput 6(9):2872–2887. https://doi.org/10.1021/ct100326h
Kesharwani MK, Brauer B, Martin JML (2015) Frequency and zero-point vibrational energy scale factors for double-hybrid density functionals (and other selected methods): can anharmonic force fields be avoided? J Phys Chem A 119(9):1701–1714. https://doi.org/10.1021/jp508422u
Arı H, Özpozan T, Büyükmumcu Z, Kabacalı Y, Saçmaci M (2016) Theoretical and vibrational spectroscopic approach to keto-enol tautomerism in methyl-2-(4-methoxybenzoyl)-3-(4-methoxyphenyl)-3-oxopropanoylcarbamate. J Mol Struct 1122:48–61. https://doi.org/10.1016/j.molstruc.2016.05.060
Roberts JD, Caserio MC (1977) Chapter 10. Alkenes and alkynes I. Ionic and radical addition reactions. In: Basic principles of organic chemistry. 2nd edn. Benjamin, Inc.: Menlo Park
Fukui K, Yonezawa T, Shingu H (1952) A molecular orbital theory of reactivity in aromatic hydrocarbons. J Chem Phys 20(4):722–725. https://doi.org/10.1063/1.1700523
Parthasarathi R, Subramanian V, Chattaraj PK (2003) Effect of electric field on the global and local reactivity indices. Chem Phys Lett 382(1–2):48–56. https://doi.org/10.1016/j.cplett.2003.09.160
Parr RG, Lv S, Liu S (1999) Electrophilicity index. J Am Chem Soc 121(9):1922–1924. https://doi.org/10.1021/ja983494x
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105(26):7512–7516. https://doi.org/10.1021/ja00364a005
Datta D (1992) On Pearson’s HSAB Principle. Inorg Chem 31(13):2797–2800. https://doi.org/10.1021/ic00039a025
Parr RG, Weitao Y (1989) Density-functional theory of atoms and molecules. International series of monographs on chemistry1st edn. Oxford University Press, New York
Sastre G, Corma A (2009) The confinement effect in zeolites. J Mol Catal A Chem 305(1–2):3–7. https://doi.org/10.1016/j.molcata.2008.10.042
Derouane EG (1987) The energetics of sorption by molecular sieves: surface curvature effects. Chem Phys Lett 142(3,4):200–204
Boekfa B, Pantu P, Probst M, Limtrakul J (2010) Adsorption and tautomerization reaction of acetone on acidic zeolites: the confinement effect in different types of zeolites. J Phys Chem C 114(35):15061–15067. https://doi.org/10.1021/jp1058947
Derouane EG, Chang CD (2000) Confinement effects in the adsorption of simple bases by zeolites. Microporous Mesoporous Mater 35–36:425–433. https://doi.org/10.1016/S1387-1811(99)00239-5
Zicovich-Wilson CM, Corma A, Viruela P (1994) Electronic confinement of molecules in microscopic pores. A new concept which contributes to the explanation of the catalytic activity of zeolites. J Phys Chem 98(42):10863–10870. https://doi.org/10.1021/j100093a030
Corma A (2003) State of the art and future challenges of zeolites as catalysts. J Catal 216(1–2):298–312. https://doi.org/10.1016/S0021-9517(02)00132-X
Kozuch S, Shaik S (2011) How to conceptualize catalytic cycles? The energetic span model. Acc Chem Res 44(2):101–110. https://doi.org/10.1021/ar1000956
Kozuch S (2012) A refinement of everyday thinking: the energetic span model for kinetic assessment of catalytic cycles. Wiley Interdiscip Rev Comput Mol Sci 2(5):795–815. https://doi.org/10.1002/wcms.1100
Carvajal MÀ, Kozuch S, Shaik S (2009) Factors controlling the selective hydroformylation of internal alkenes to linear aldehydes. 1. The isomerization step. Organometallics 28(13):3656–3665. https://doi.org/10.1021/om801166x
Meek SJ, Pitman CL, Miller AJM (2016) Deducing reaction mechanism: a guide for students, researchers, and instructors. J Chem Educ 93(2):275–286. https://doi.org/10.1021/acs.jchemed.5b00160
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 109 kb)
Rights and permissions
About this article
Cite this article
Ghambarian, M., Ghashghaee, M., Azizi, Z. et al. Structural diversity of metallacycle intermediates for ethylene dimerization on heterogeneous NiMCM-41 catalyst: a quantum chemical perspective. Struct Chem 30, 137–150 (2019). https://doi.org/10.1007/s11224-018-1184-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-018-1184-3