Abstract
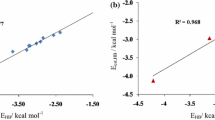
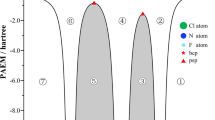
Density functional theory (DFT) and ab initio calculations were performed for difluoroacetic acid (DFA). Eight theoretically possible conformers were considered and by using conformational analysis only three stable conformers were found. The hydrogen bonding interaction of DFA complex has been investigated using DFT and ab initio methods for cis conformers. Stabilization energies of dimers including basis set superposition error and ZPE were found in the range 8.89–13.08 kcal mol−1. It was found that EFC dimer is slightly more stable. Red shift of O–H bond in the range −226.3 to 505.7 cm−1 predicted for dimers. The natural bond orbital analysis was applied to characterize nature of the interaction.




Similar content being viewed by others
References
Scheiner S (1997) Hydrogen bonding. Oxford University Press, New York
Jeffery GA (1997) An introduction to hydrogen bonding. Oxford University Press, New York
Jeffrey GA, Saenger W (1991) Hydrogen bonding in biological systems. Springer-Verlag, Berlin
Hadzi D (1997) Theoretical treatments of hydrogen bonding. Wiley, Chichester
Muller-Dethlefs K, Hobza P (2000) Chem Rev 100:143–167
Hobza P, Havlas Z (2000) Chem Rev 100:4253–4264
Hobza P (2002) Int J Quantum Chem 90:1071–1074
Karpfen A, Kryachko ES (2005) Chem Phys 2005(310):77–84
Grabowski SJ (2006) Hydrogen bonding—new insights. Springer, Berlin
Steiner T (2002) Angew Chem Int Ed 41:48–76
Bijen JMJM, Derrissen JL (1975) J Mol Struct 27:233–240
Van Eijck BP, Maagdnberg AA, Janssen G, Van Goethem-Wiersma TG (1983) J Mol Struct 98:282–303
De With G (1983) J Mol Struct 18:241–245
Hartree R (1927) Proc Camb Philos Soc 24:89–110
Fock V (1930) Z Phys 61:126–148
Slater JC (1930) Phys Rev 35:210–211
Møller C, Plesset MS (1934) Phys Rev 46:618–622
Parr RG, Yang W (1994) Density-functional theory of atoms and molecules. Oxford University Press, New York
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Becke AD (1993) J Chem Phys 98:1372–1377
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala Y, Cui Q, Morokuma K, Rega N, Salvador P, Dannenberg JJ, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres J, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (1998) GAUSSIAN 98, Revision A.7, Gaussian, Inc., Pittsburgh, PA
Head-Gordon M, Replogle ES, Pople JA, Boys SF, Bernardi F (1970) Mol Phys 19:553–566
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Weinhold F (1992) NBO Version 3.1. Theoretical Chemistry Institute, University of Wisconsin, Madison
Chermahini AN, Ghaedi A, Teimouri A, Momenbeik F, Dabbagh HA (2008) J Mol Struct (Theochem) 867:78–84
Chocholousova J, Vacek J, Hobza P (2003) J Phys Chem 107:3086–3092
Anderson MP, Uvdal P (2005) J Phys Chem A 109:2937–2941
NIST Chemistry WebBook, NIST Standard Reference Database, Number 69, June 2005 Release. http://webbook.nist.gov/chemistry/
Acknowledgment
This study was financially supported by Yasouj University (YU).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chermahini, A.N., Moaddeli, A. & Teimouri, A. Ab initio and DFT studies of hydrogen bond interactions in difluoroacetic acid dimer. Struct Chem 21, 643–649 (2010). https://doi.org/10.1007/s11224-010-9595-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-010-9595-9