Abstract
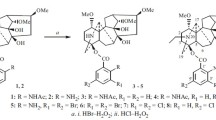
Bromination of lappaconitine with a KBr—H2O2 mixture in 20% H2SO4, as well as with molecular Br2 in either Py, CH2Cl2, CH3COOH, or DMSO did not occur, while the initial lappaconitine was isolated from the reaction mixture. Such a bromination with molecular Br2 in solutions of strong acids (concentrated HCl, aqueous 50% H2SO4, and CF3COOH) gives monobromolappaconitine in the yields up to 80%. Bromination of N-desacetyllappaconitine both in organic solvents and in acid solutions leads to mono- and dibrominated derivatives.
Similar content being viewed by others
References
S. F. Sokolov, Allapinin i sovremennyye podkhody k lecheniu narushenii ritma serdtsa (metodicheskie rekomendatsii po primeneniu preparata) [Allapinin and Modern Approaches to the Treatment of Cardiac Arrhythmias (Guidelines for the Drug Administration)], FGU RKNPK Rosmedtechnologii, Moscow, Russian Federation, 2011, p. 35 (in Russian).
S. F. Sokolov, Vestn. aritmologii [Journal of Arrhythmology], 2011, 64, 60 (in Russian).
M. S. Yunusov, Russ. Chem. Bull., 2011, 60, 633.
A. A. Akhiyarov, A. N. Lobov, S. P. Ivanov, L. V. Spirikhin, T. M. Gabbasov, E. M. Tsyrlina, N. V. Valiev, A. Z. Sadikov, S._S. Sagdullaev, M. S. Yunusov, Russ. Chem. Bull., 2020, 69, 567.
Pat. RU 2 295 524 C1; Bull. Izobret. [Inventions. Utility Models], 2007, No. 8, 7 (in Russian).
S. A. Osadchii, Dr. Sci. Chem. Thesis, N. N. Vorozhtsov Novosibirsk Institute of Organic Chemistry, Siberian Branch of Russian Academy of Science, Novosibirsk, Russian Federation, 2008, p. 220 (in Russian).
T. G. Tolstikova, E. E. Shults, A. O. Bryzgalov, M. V. Khvostov, V. E. Romanov, S. A. Osadchii, G. A. Tolstikov, Chem. Sustainable Development, 2007, 15, 591.
Q. H. Chen, F. P. Wang, Chin. Chem. Lett., 2001, 12, 421.
V. G. Kasradze, I. B. Ignatyeva, R. A. Khusnutdinov, K. Yu. Suponitskii, M. Yu. Antipin, M. S. Yunusov, Chem. Heterocycl. Compd. (Engl. Transl.), 2012, 48, 1018.
T. M. Gabbasov, E. I. Andrianova, S. F. Petrova, S. P. Ivanov, E. M. Tsyrlina, A. Z. Sadikov, S. S. Sagdullaev, M. S. Yunusov, Chem. Nat. Compd. (Engl. Transl.), 2020, 56, 767; DOI: https://doi.org/10.1007/s10600-020-03145-5.
E. G. Zinurova, N. N. Kabal’nova, V. V. Shereshovets, E. V. Ivanova, E. E. Shults, G. A. Tolstikov, M. S. Yunusov, Russ. Chem. Bull., 2001, 50, 720.
O. Achmatowicz, Jr, Y. Tsuba, L. Marion, Can. J. Chem., 1965, 43, 2336.
K. Ingold, Structure and Mechanism in Organic Chemistry, Cornell University press Ithaca and London, London, 1969.
N. P. Kanyaev, E. A. Shilov, Dokl. AN SSSR, 1939, 24, 891 (in Russian).
M. Smit, Organicheskaya khimiya Marcha [March’s Organic Chemistry], Laboratoria znanii, Moscow, Russian Federation, 2020, Vol. 2, p. 299 (in Russian).
W. Gottardi, Monatsh. Chem., 1969, 100, 42.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was financially supported by the Russian Science Foundation (Project No. 19-13-00096, 2019 Competition “Conducting Fundamental Scientific Research and Exploratory Research by Individual Scientific Groups”).
This paper does not contain descriptions of studies on animals or humans.
The authors declare no conflict of interest, financial or otherwise.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 3, pp. 515–519, March, 2021.
Rights and permissions
About this article
Cite this article
Chernikova, I.B., Gabbasov, T.M., Tsyrlina, E.M. et al. Bromination of lappaconitine and N-desacetyllappaconitine. Russ Chem Bull 70, 515–519 (2021). https://doi.org/10.1007/s11172-021-3117-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3117-3