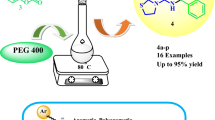
Abstract
A facile and efficient greener synthesis of a novel four-fused ring heterocyclic system via three-component reactions of [1,3,4]thiadiazolo[3,2-a]pyrimidin-5-one, aromatic aldehyde, and 1,3-cyclohexanediones, in the presence of 1-(4-sulfonic acid)butyl-3-methylimidazolium hydrogen sulfate, has been presented. The synthesized compounds were characterized by mass spectrometry, IR, 1H-NMR, 13C-NMR, and elemental analysis. The remarkable features of this method are using acidic ionic liquid catalyst, green solvent media, short reaction time, good yields, and simple purification techniques.
Graphical abstract




Similar content being viewed by others
References
N. Pathan, P. Ali, A. Rahatgaonkar, K. Al-Mousa, Heterocycl. Chem. 58, 1675 (2021)
R. Olar, M. Badea, C. Maxim, A.M. Grumezescu, C. Bleotu, L. Măruţescu, M.C. Chifiriuc, Materials. 13, 4640 (2020)
G.P. Raju, S. Gugulothu, K.S. Syed, Caribb. J. Sci. Technol. 7, 015 (2019)
S. Janowska, A. Paneth, M. Wujec, Molecules 25, 4309 (2020)
B. Sravanthi, L. Kaviarasan, T.K. Praveen, P.S. SaiKiran, C. Pavankumar, B. Gowramma, J. Iran. Chem. Soc. 17, 2359 (2020)
K. Savateev, V. Fedotov, I. Butorin, O. Eltsov, P. Slepukhin, E. Ulomsky, V. Rusinov, R. Litvinov, D. Babkov, E. Khokhlacheva, P. Radaev, P. Vassiliev, A. Spasov, Eur. J. Med. Chem. 185, 111808 (2020)
S.V. Tiwari, J.A. Seijas, M.P. Vazquez-Tato, A.P. Sarkate, D.K. Lokwani, A.P.G. Nikalje, Molecules 21, 894 (2016)
G.S. Khansole, J.A. Angulwar, V.N. Bhosale, S.S. Choudhare, Int. J. Res. Anal. Rev. 6, 525 (2019)
M.E. Azab, S.S. Abdel-Wahab, N.F. Mahmoud, G.A. Elsayed, J. Heterocycl. Chem. 55, 2349 (2018)
J.A. Angulwar, G.S. Khansole, V.N. Bhosale, Asian j. Org. Med. Chem. 4, 40 (2019)
N.S. El-Sayed, E.R. El-Bendary, S.M. El-Ashry, M.M. El-Kerdawy, Eur. J. Med. Chem. 46, 3714 (2011)
M.F. Said, H.H. Georgey, E.R. Mohammed, Eur. J. Med. Chem. 224, 113682 (2021)
K. Eskandari, Y. Pourshojaei, F. Haghani, M. Shabani, A. Asadipour, Heliyon. 5, e02426 (2019)
M. Biglari, F. Shirini, N.O. Mahmoodi, M. Zabihzadeh, M. Mashhadinezhad, J. Mol. Struct. 1205, 127652 (2020)
T. Raj, R.K. Bhatia, M. Sharma, A.K. Saxena, M.P.S. Ishar, Eur. J. Med. Chem. 45, 790 (2010)
N. Mulakayala, P.V.N.S. Murthy, D. Rambabu, M. Aeluri, R. Adepu, G.R. Krishna, C.M. Reddy, K.R.S. Prasad, M. Chaitanya, C.S. Kumar, M.V.B. Rao, M. Pal, Bioorganic Med. Chem. Lett. 22, 2186 (2012)
S.P. Khare, T.R. Deshmukh, S.V. Akolkar, J.N. Sangshetti, V.M. Khedkar, B.B. Shingate, Res. Chem. Intermed. 45, 5159 (2019)
P.K. Paliwal, S.R. Jetti, S. Jain, Med. Chem. Res. 22, 2984 (2013)
K. Hiramoto, A. Nasuhara, K. Michikoshi, T. Kato, K. Kikugawa, Mutat. Res. -Genet. Toxicol. Environ. Mutagen. 395, 47 (1997)
T.C. Raveesha, M.K. Hema, K.J. Pampa, P.G. Chandrashekara, K. Mantelingu, T. Demappa, N.K. Lokanath, J. Mol. Struct. 1225, 129104 (2021)
M.N. Erichsen, T.H.V. Huynh, B. Abrahamsen, J.F. Bastlund, C. Bundgaard, O. Monrad, A. Bekker-Jensen, C.W. Nielsen, K. Frydenvang, A.A. Jensen, L. Bunch, J. Med. Chem. 53, 7180 (2010)
A. Kulshrestha, G.K. Katara, S.A. Ibrahim, R. Patil, S.A. Patil, K.D. Beaman, Oncotarget 8, 67017 (2017)
M. Nagamani, P. Jalapathi, B. Shankar, M. Neelamma, T.M. Krishna, Russ. J. Gen. Chem. 89, 998 (2019)
D. Kumar, V.B. Reddy, S. Sharad, U. Dube, S. Kapur, Eur. J. Med. Chem. 44, 3805 (2009)
K. Niknam, A. Piran, Green Sustain. Chem. 3, 8 (2013)
P. Chavan, D. Pansare, R. Shelke, S. Shejul, P. Bhoir, Curr. Chem. Lett. 10, 43 (2021)
A. Shaabani, M.M. Amini, S. Ghasemi, R. Ghadari, A.H. Rezayan, Y. Fazaeli, S. Feizi, Chem. Pharm. Bull. 58, 270 (2010)
X. Zhang, W. Xiong, M. Shi, Y. Wu, X. Hu, Chem. Eng. J. 408, 127866 (2021)
A.J. Greer, J. Jacquemin, C. Hardacre, Molecules 25, 5207 (2020)
Z. Yang, C. Sun, C. Zhang, S. Zhao, M. Cai, Z. Liu, QYu. Tribol, Int. 153, 106663 (2021)
P. Halder, S. Kundu, S. Patel, A. Setiawan, R. Atkin, R. Parthasarthy, J. Paz-Ferreiro, A. Surapaneni, K. Shah, Renew. Sust. Energ. Rev. 105, 268 (2019)
I. Wazeer, M.K. Hadj-Kali, I.M. AlNashef, Applications of ionic liquids and deep eutectic solvents in biorefinery-biodiesel production (Springer, Cham, 2019), p.185
Z. Rahmatizadeh-Pashaki, N. Daneshvar, F. Shirini, J. Iran. Chem. Soc. 18, 2135 (2021)
S. Sadjadi, F. Koohestani, J. Mol. Liq. 319, 114393 (2020)
Y. Wang, J. Zhou, K. Liu, L. Dai, RSC Adv. 3, 9965 (2013)
T. Welton, Chem. Rev. 99, 2071 (1999)
A.S. Amarasekara, Chem. Rev. 116, 6133 (2016)
M.B. Martini, M.V. Bravo, C.G. Adam, Chem. Proc. 3, 127 (2021)
B. Han, F. Yin, S. Liu, X. Zhao, J. Liu, C. Wang, H. Yang, W. Zhang. Int. J. Chem. React. Eng. 17 (2019)
S.K. Singh, A.W. Savoy, J. Mol. Liq. 297, 112038 (2020)
C.G. Neochoritis, T. Zarganes-Tzitzikas, K. Katsampoxaki-Hodgetts, A. Dömling, J. Chem. Educ. 97, 3739 (2020)
D. Insuasty, J. Castillo, D. Becerra, H. Rojas, R. Abonia, Molecules 25, 505 (2020)
H.A. Younus, M. Al-Rashida, A. Hameed, M. Uroos, U. Salar, S. Rana, K.M. Khan, Expert. Opin. Ther. Pat. 31, 267 (2021)
S. Das, RSC Adv. 10, 18875 (2020)
X. Wang, B. Zhu, J. Dong, H. Tian, Y. Liu, H. Song, Q. Wang, Chem. Commun. 57, 5028 (2021)
J. Fairoosa, S. Saranya, S. Radhika, G. Anilkumar, ChemistrySelect 5, 5180 (2020)
C. Lamberth, Bioorg. Med. Chem. 28, 115471 (2020)
K.K. Das, S. Manna, S. Panda, Chem. Commun. 57, 441 (2021)
M.A. Ghasemzadeh, B. Mirhosseini-Eshkevari, M. Tavakoli, F. Zamani, Green Chem. 22, 7265 (2020)
P.S.G. Nunes, H.D.A. Vidal, A.G. Corrêa, Org. Biomol. Chem. 18, 7751 (2020)
M. Esmaeilinezhad, A.A. Esmaeili, S. Jannati, J. Chem. Res. 42, 618 (2018)
A.A. Esmaeili, F. Mesbah, A. Moradi, A. Khojastehnezhad, M. Khalili, Phosphorus Sulfur Silicon Relat. Elem. 196, 819 (2021)
A.A. Esmaeili, F. Mesbah, M. Zangouei, S. Amini-Ghalandarabad, M. Tasmimi, A. Habibi, A.R. Fakhari, A. Khojastehnezhad, Res. Chem. Intermed. 47, 3537 (2021)
A. Sethiya, J. Soni, A. Manhas, P.C. Jha, S. Agarwal, Res. Chem. Intermed. 47, 4477 (2021)
S. Hosseini, A.A. Esmaeili, A. Khojastehnezhad, B. Notash, J. Sulphur Chem. 42, 628 (2021)
P.V. Bhongale, S.S. Joshi, N.A. Mali, Chem. Pap. 74, 4461 (2020)
Acknowledgements
The Research Department of the Ferdowsi University of Mashhad is acknowledged for financial support (Grant No. 3/56584).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
EM done investigation; validation; and writing-original draft preparation. AAE did project administration, supervised the work, revised, and completed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This declaration is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mahdavi, E., Esmaeili, A.A. Efficient synthesis of novel chromeno[2,3-d][1,3,4]thiadiazolo[3,2-a]pyrimidine derivatives via three-component reaction using acidic ionic liquid catalysts in ethylene glycol. Res Chem Intermed 49, 1297–1310 (2023). https://doi.org/10.1007/s11164-022-04944-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04944-x