Abstract
In this paper, calcium iodate nanoparticles were first synthesized by the modified reaction of Ca(NO3)2 and KIO3 in an aqueous medium under ultrasonic irradiation. The structure of nanocatalyst was then characterized by FT-IR, FESEM, EDX, XRD, and BET techniques. Afterward, the fabricated Ca(IO3)2 was applied as a nanocatalyst in the facile synthesis of heterocycles including quinoxalines, 5,6-dicyano pyrazines, and pyrido[2,3-b]pyrazines. For this purpose, the feasibility of the reaction in the presence of different catalyst amounts, solvents, and temperatures was first investigated. Next, the target compounds were obtained by the condensation reaction of aryl-1,2-diamines or 2,3-diaminomaleonitrile with 1,2-diketones in the presence of a catalytic amount of Ca(IO3)2 in ethanol or acetic acid solvents at ambient temperature in good to excellent yields. One of the salient advantages of this work is the synthesis of calcium iodate nanoparticles by chemical precipitation method and its application as a heterogeneous nanocatalyst for the first time in the synthesis of organic compounds. The other important benefits of this process are the use of an inexpensive, safe, stable and recyclable catalyst, high product yields, short reaction times, and easy isolation of the product in pure form.
Similar content being viewed by others
Introduction
Although quinoxaline derivatives have long been known, they is still of great significance for the chemical industry researchers due to their ever-increasing applications in dyes, drugs, and electrical/photochemical compounds [1,2,3,4,5,6,7,8]. One of the interesting applications of these derivatives is the presence of quinoxaline or pyrazine ring in the structure of drugs such as equinomycin, actinolutein (antibiotic) [9, 10], carbadox (anti-pig dysentery), amiloride (for the treatment of high blood pressure), morinamide (anti-tuberculosis), favipiravir (antivirus recently proposed for the treatment of corona disease or Covid-19), and glipizide (anti-diabetes) (Fig. 1).
Nowadays, given the worldwide spread of coronavirus and the presence of pyrazine ring in antivirus drugs such as favipiravir, the importance and necessity of the development of novel catalytic methods for the facile synthesis of these compounds in addition to conventional methods have multiplied. A number of methods for the synthesis of these compounds through the condensation of aryl-1,2-diamines with 1,2-diketones under reflux conditions or in the presence of an acidic catalyst under various conditions have already been reported. A number of catalysts including molecular iodine [11], ceric (IV) ammonium nitrate [12], polyaniline sulfate salt [13], montmorillonite K-10 [14], gallium triflate [15], zeolite-nickel [16], g-C3N4/Cu3TiO4 [17], ionic liquid-functionalized chitosan [DSIM][AlCl3]x–@CS [18], poly(Ani‑co‑Py)@CNT‑Fe3O4 [19], and so on [20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] have been reported for the preparation of these compounds. In addition, some catalytic methods have been reported for the synthesis of 5,6-dicyanopyrazines [36,37,38,39]. However, despite some advantages, each of these methods suffers from a number of drawbacks. Thus, the development of novel methods or further attempts to overcome these disadvantages are still in demand.
Calcium, which is one of the most abundant elements in the world, is present in the form of known compounds such as calcium chloride, phosphate, carbonate, silicate, oxide, sulfate, and hydroxide salts [40,41,42]. Although many efforts have been made in the previous decades on the preparation and application of these compounds in different processes, few research studies have been performed on calcium iodate. However, it must be pointed out that calcium iodate has been applied as an additive in various industries, including poultry, medicine, and deodorants [43,44,45,46]. This salt (CI) is also the most common source of iodine and is safer and more stable than other iodine sources. The most abundant anhydrous calcium iodate ore in the nature is known as lautarite, which is also the most important source of iodine [47]. Metal iodates such as AgIO3, Ca(IO3)2, Mn(IO3)2, and Cu(IO3)2 are usually synthesized by chemical precipitation method, which forms large sized particles. Therefore, no very fine iodine particles are commercially available [48]. Different metal iodate nanoparticles have recently been prepared by mechanochemical single diffusion methods. The corresponding metal nitrates and potassium iodate have been prepared under solvent-free conditions. Metal iodates are much stronger oxidizing agents for thermal applications compared with metal oxides due to the formation of the corresponding metal oxides as well iodine gas at relatively low temperatures following decomposition [49]. However, to the best of our knowledge, there have not been reports concerning the synthesis of calcium iodate nanoparticles by chemical precipitation under ultrasonic irradiation and its application as a heterogeneous catalyst in the facile synthesis of organic compounds. Therefore, in continuation of our current work on the synthesis of solid catalysts and their application in the development of novel methods for the formation of important heterocyclic compounds [50,51,52], we report the preparation of calcium iodate nanoparticles for the first time and their application in an efficient and eco-friendly catalytic method for the synthesis of quinoxalines, pyridopyrazines, and 5,6-dicyano pyrazines through the one-pot condensation of aryl-1,2-diamines or 2,3-diaminomaleonitrile with 1,2-diketone under green conditions.
Experimental
Chemicals and apparatus
Melting points were determined using a Barnstead Electrothermal 9200 apparatus, and they may be uncorrected. 1H NMR and 13C NMR spectra were recorded on a Bruker spectrophotometer (300 and 500 MHz) in DMSO-d6 solvent using Me4Si as the internal standard. FT-IR spectra were recorded using a JASCO FT-IR 4200-A spectrophotometer. The mass spectra were recorded on an Agilent model 5975C VL MSD spectrometer with a triple-axis detector spectrometer at 70 eV. The shape, size, and atom type of the nanoparticles were investigated by SEM and EDX images recorded using a Philips XL30 instrument. Nitrogen adsorption and desorption isotherms (BET analysis) were recorded at − 196 °C by a Belsorb II system (Japan) after the samples were vacuum dried at 150 °C overnight. The progress of the reactions was routinely monitored by thin-layer chromatography on silica gel F254 aluminum sheets (Merck). All obtained commercially materials were used without further purification.
Preparation of nano-Ca(IO3)2
To a 50-ml beaker containing 10 ml of distilled water and Ca(NO3)2. 4H2O, (2.36 gr, 1 mmol) was added an aqueous solution of KIO3 (4.28 gr, 2 mmol) and the mixture obtained was subjected to ultrasonication for 10 min. The reaction mixture was stirred for 2 h at room temperature until a white precipitate of calcium iodate salt (3.81 gr, 98%) formed, which was then filtered, washed with water, and dried at 110 °C. Ca(IO3)2 (1 g) was subjected to ultrasonic irradiation for 1 h to provide nanosized particles. The nanocatalyst was then used without further purification.
Typical procedure for preparation of compounds (3a–q)
1,2-Arylenediamine, 1a–d, or 2,3-diaminomaleonitrile, 1e, (0.1 mmol) and the corresponding 1,2-diketones (0.1 mmol), 2a–d, were dissolved in ethanol by continuous stirring. A catalytic amount (3 mol%, 0.005 gr) of Ca(IO3)2 was added to the solution. The reaction mixture was then stirred at room temperature for 3–20 min (Tables 2 and 3). The reaction progress was monitored by TLC. After completion of the reaction, the used catalyst was collected by filtration and cold water was added to the filtrate to obtain the product. Afterward, the residue was filtered and washed with cold ethanol/water to yield compounds 3a–q. In this method, pure products were obtained. For further purifications, the crude products were recrystallized from EtOH or MeOH/AcOH mixtures.
Spectroscopic data for selected compounds
2,3-Diphenyl quinoxaline (3a): FT-IR (KBr, υmax): 3058 (CH), 1558, 1548 (C=N), 1477, 1440, 1395, 1345, 1247, 1218 (C=C), 1128, 1076, 1057 (C–N), 977, 770, 698, 598, 539 cm−1; 1H NMR (500 MHz, CDCl3) δH: 7.32–7.37 (m, 6H, H–Ar), 7.53 (dd, J = 1.45 Hz, J = 6.26 Hz, 4H, H–Ar), 7.77 (dd, J = 3.45 Hz, J = 3.01 Hz, 2H, H–Ar), 8.19–8.17 (m, 2H, H–Ar) ppm; 13C NMR (125 MHz, CDCl3) δC: 153.5, 141.2, 139.0, 129.9, 129.8, 129.2, 128.8, 128.3 ppm.
Acenaphtho[1,2-b]pyrido[2,3-e]pyrazine (3 k): FT-IR (KBr, υmax): 3051 (C-H), 2371 (CN), 1612, 1562 (C=N), 1527, 1489, 1433, 1294, 1212 (C=C), 1097, 1034 (C–N), 827, 772, 426 cm−1; 1H NMR (300 MHz, CDCl3) δH: 7.70–7.74 (m, 1H, H–Ar), 7.83 (q, J = 2.40 Hz, 2H, H–Ar), 8.13 (dd, J = 2.55 Hz, J = 5.70 Hz, 2H, H–Ar), 8.40 (d, J = 6.90 Hz, 1H, H–Ar), 8.52 (t, J = 7.12 Hz, 2H, H–Ar), 9.12 (d, J = 4.20 Hz, 1H, H–Ar) ppm; 13C NMR (75 MHz, CDCl3) δC: 122.3, 123.3, 124.3, 128.7, 129.9, 130.0, 130.2, 130.9, 131.2, 136.4, 137.2, 150.7, 152.4, 155.0, 157.1 ppm.
5,6-Diphenylpyrazine-2,3-dicarbonitrile (3 m): FT-IR (KBr, υmax): 3067 (CH), 2348 (C≡N), 1660 (C=N), 1375–1579 (C=C), 831 cm−1; 1H NMR (300 MHz, DMSO- d6), δH: 7.20 (d, J = 6.90 Hz, 2H, H-ph), 7.28 (d, J = 7.29 Hz, 2H, H-ph), 7.40–7.59 (m, 4H, H-ph), 7.72 (d, J = 7.42 Hz, 2H, H-ph) ppm; 13C NMR (75 MHz, DMSO- d6) δC: 129.0, 130.1, 130.5, 131.0, 132.6, 135.9, 155.2 ppm.
Results and discussion
Catalyst characterization
Calcium iodate (Ca(IO3)2) nanocatalyst was first prepared by a modified method through the reaction of potassium iodate and calcium nitrate in an aqueous medium in an ultrasonic bath [45] (Scheme 1). Since the investigation of the structure and configuration of nanocatalysts is an important parameter in predicting their catalytic behavior, the structure and morphology of Ca(IO3)2 nanoparticles were studied by conventional characterization techniques and different analytical methods.
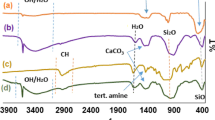
The FT-IR spectrum of calcium iodate is shown in Fig. 2. The bands observed at 1608 and 3371 cm−1 correspond to the stretching and bending vibrations of the O–H present or adsorbed in the salt structure. The relatively strong vibrating band at 3463 cm−1 may be associated with the hydrogen bond of the water molecule. The two bands at 589 and 816 cm−1 in addition to those at 665 and 779 cm−1 can be ascribed to the symmetric and asymmetric stretching and bending vibrations of iodate ion. Finally, the infrared fundamental frequencies, corresponding to metal, iodine, and oxygen atoms, are mostly observed in the range of 800–400 cm−1 , confirming the crystalline structure of the salt [45, 46].
Figure 3 shows the FESEM images of Ca(IO3)2 nanoparticles, which provide valuable data regarding the particle size and morphology. The particle sizes were mostly in the 24–52 nm range. In addition, the crystals seem to be cubic.
EDX analysis was used for the qualitative and quantitative determination of the elements present in the salt structure. The specific weights and percentages of oxygen, calcium, and iodine are observed in Fig. 4.
The N2 adsorption/desorption isotherms of Ca(IO3)2 nanoparticles are shown in Fig. 5a. A type II isotherm salt structure with a small type H1 hysteresis loop in the range of 0.3–0.9 p/p° was observed, indicating non-porous microframeworks [53, 54]. The structural data indicate that the adsorption surface area (BET), total pore volume, and mean pore diameter of Ca(IO3)2 are 61 m2/g, 0.009 cm3/g, and 11.01 nm, respectively. Furthermore, the pore distribution curve (BJH) shown in Fig. 5b indicates that the pore radii are mostly in the range of 10 nm.
XRD analysis was carried out in order to determine the crystal structure of nanocalcium iodate. As observed in Fig. 6, the 2θ values appearing at 32.28°, 46.1°, 54.8°, 57.48°, 67.40°, 76.32°, and 85.12° relatively match the calcium standards (JCDPS card no. 96-900-8465). This structure can be characterized as orthorhombic (space group Fdd2(43)-ICDD: 01-076-2134), respectively. In addition, the size of the nanoparticles is estimated to be about 50 nm using the Scherer's equation [46].
Catalytic activity
Having determined the structure of calcium iodate nanoparticles prepared, its catalytic performance in the one-pot synthesis of quinoxalines, pyridopyrazines, and cyano-substituted pyrazines was studied. To determine the optimal conditions, the effects of solvent and catalyst amount on the reaction yield, time, and temperature were investigated and the corresponding results are shown in Table 1. As the results indicate, in the synthesis of quinoxaline 3a through the reaction of ortho phenylenediamine with benzyl (model reaction), the application of 3 mol% of the catalyst and ethanol solvent gave the highest product yield (97%) at 25 °C and of 3 min, respectively (Table 1, entry 3). Further increasing the amount of the catalyst and reaction time did not considerably improve the reaction conditions. In addition, in the reaction of 2,3-diaminopyridine with benzyl under optimal conditions, the yield slightly decreased (Table 2, entry 9).
This method was next applied in the reaction of diaminomaleonitrile with benzyl under optimal conditions to prepare 2,3-dicyanopyrazine, 3 m. Despite the presence of electron-withdrawing groups on 2e, which can make the reaction difficult, the product was obtained in high yield under moderate reaction conditions using 3 mol% of the catalyst and acetic acid solvent (Table 3, entry 1). In order to show the range and performance of the nanocatalyst under optimal conditions, the facile synthesis of quinoxaline derivatives, pyrido[2,3-b]pyrazine, and 2,3-dicayanopyrazines through the one-pot reaction of aryl-1,2-diamines or 2,3-diaminomaleonitrile with 1,2-diketones was successfully carried out (Scheme 2). The corresponding results are shown in Tables 2 and 3.
According to the results in Table 2, the presence of an electron withdrawing substituent (NO2) on the phenylenediamine ring considerably deceased the reaction yield, unlike an electron donating substituent (CH3). In addition, as shown in Table 3, the presence of two electron-withdrawing groups in the structure of diaminomaleonitrile makes the reaction more difficult. Nevertheless, several tests showed that the best reaction yield (95%, Table 3, entry 1) was still obtained by using 3% mol of the nanocatalyst, room temperature, and acetic acid solvent. However, all products (3a–q) are known compounds and their physical and spectroscopic data match those reported in the literature [50,51,52,53].
Scheme 3 shows the proposed mechanism for the synthesis of compounds 3a–q. Calcium ion first activates the carbonyl group of 1,2-diketone as a Lewis acid to form intermediate (A), following the nucleophilic attack of the amine group. Schiff base (B) is then generated through the catalytic oxidation and removal of a water molecule. Finally, the intramolecular nucleophilic attack of the second amine substituent and the oxidative elimination of the second water molecule yield quinoxaline, pyrido[2,3-b]pyrazine, and 3,4-dicyanopyrazine heterocycle rings (3a–q) through the catalytic activation of the imine carbon. The shortening of the reaction time and the increased yield compared to previous reports, in addition to the presence of calcium ions as a strong Lewis acid, may be due to the higher oxidizing effect of iodate ions.
The recyclability of the heterogeneous catalyst in the model reaction was investigated under optimal conditions. Upon completion of the reaction, the calcium iodate nanocatalyst was easily removed from the reaction mixture by filtration, washed several times with ethanol, dried in a vacuum oven, and reused in the subsequent runs. Table 4 shows the results of catalyst recyclability tests. No significant loss was observed in the catalytic activity after 9 cycles.
FT-IR spectroscopy was used to confirm the structure of the recovered catalyst. As observed in Fig. 7, there are almost no differences between the FT-IR spectra of the fresh and recovered calcium iodate catalysts.
A comparison Ca(IO3)2 nanocatalyst and some previous catalysts is listed in Table 5, revealing that this catalyst is better to other reported catalysts in terms of yield, amount of catalyst, and reaction time.
Conclusion
Calcium iodate salt nanoparticles were synthesized and characterized for the first time by the facile method of chemical precipitation under ultrasonic irradiation. This nanostructured salt was then used as a mild and high-performance catalyst for the facile synthesis of quinoxaline, pyrido[2,3-b]pyrazine, and 3,4-dicyanopyrazine derivatives in EtOH or AcOH at ambient temperature. According to the results obtained, this procedure is simple and the products are easily separated.
Other attractive features of the method including good conversion, safety, and recyclability of the catalyst make it useful for the facile preparation of target compounds.
Data availability
No data were used for the research described in the manuscript.
References
V.A. Mamedov, Quinoxalines (Springer, AG Switzerland, 2016)
M. Loriga, S. Piras, P. Sanna, G. Paglietti, Farmaco 52, 157 (1997)
X. Hui, J. Desrivot, C. Bories, P.M. Loiseau, X. Frank, R. Hocquimiller, B. Figadère, Med. Chem. Lett. 16, 815 (2006)
G. Sakata, K. Makino, Y. Karasawa, Heterocycles 27, 2481 (1988)
R. Sarges, H.R. Howerd, R.C. Browne, L.A. Label, P.A. Seymour, J. Med. Chem. 33, 2240 (1990)
S. Dailey, J.W. Feast, R.J. Peace, I.C. Sage, S. Till, E.L. Wood, J. Mater. Chem. 11, 2238 (2001)
D. O’Brien, M.S. Weaver, D.G. Lidzey, D.D.C. Bradley, Appl. Phys. Lett. 69, 881 (1996)
K.R.J. Thomas, M. Velusamy, J.T. Lin, Y.-T. Tao, C.-H. Chuen, Chem. Mater. 17, 1860 (2005)
A. Dell, D.H. William, H.R. Morris, G.A. Smith, J. Feeney, G.C.K. Roberts, J. Am. Chem. Soc. 97, 2497 (1975)
C. Bailly, S. Echepare, F. Gago, M. Waring, J. Anticancer Drug Des. 15, 291 (1999)
J. Guillon, G. Philippe, M. Labaied, P. Sonnet, J.M. Lèger, P.D. Poulain, I.F. Bares, P. Dallemagne, N. Lemaitre, F. Pehourcq, J. Rochette, C. Sergheraert, J. Christian, J. Med. Chem. 47, 1997 (2004)
R.S. Bhosale, S.R. Sarda, S.S. Ardhapure, W.N. Jadhav, S.R. Bhusare, R.P. Pawar, Tetrahedron Lett. 46, 7183 (2005)
S.V. More, M.N.V. Sastry, C.F. Yao, Green Chem. 8, 91 (2006)
C. Srinivas, C.N.S.S.P. Kumar, V.J. Rao, S. Palaniappan, J. Mol. Catal. A. Chem. 265, 227 (2007)
T.K. Huang, R. Wang, L. Shi, X.X. Lu, Catal. Commun. 9, 1143 (2008)
M. Kalhor, Z. Seyedzade, Iran. J. Chem. Chem. Eng. 38, 27 (2019)
M. Arunachalapandi, S.M. Roopan, Res. Chem. Intermed. 47, 3363 (2021)
M.U. Khan, S. Siddiqui, Z.N. Siddiqui, ACS Omega 4(4), 7586 (2019)
S.F. Hojati, A. Amiri, E. Fardi, M. Mahamed, Res. Chem. Intermed. 48, 1347 (2022)
J.J. Cai, J.P. Zou, X.Q. Pan, W. Zhang, Tetrahedron Lett. 49, 7386 (2008)
M.M. Heravi, M.H. Tehrani, K. Bakhtiari, H.A. Oskooie, Catal. Commun. 8, 1341 (2007)
M.R. Poor Heravi, F. Norouzy, Res. Chem. Intermed. 43, 4265 (2017)
K. Aghapoor, H.R. Darabi, F. Mohsenzadeh, Y. Balavar, H. Daneshyar, Transit. Met. Chem. 35, 49 (2010)
G. Rezanejade, R. Malakooti, F. Jami, Z. Parsaei, H. Atashin, Catal. Commun. 27, 49 (2012)
P.V. Shitre, R.R. Harale, B.R. Sathe, M.S. Shingare, Res. Chem. Intermed. 43, 829 (2017)
A. Hasaninejad, A. Zare, M.A. Zolfigol, M. Shekouhy, Synth. Commun. 39, 569 (2009)
V. Kunkuma, L.A.P. Devi Bethala, Y. Bhongiri, B.N.P. Rachapudi, S.S.P. Potharaju, Eur. J. Chem. 2, 495 (2011)
M. Esmaeilpour, A.R. Sardarian, Green Chem. Lett. Rev. 7, 301 (2014)
C.S. Cho, W.X. Ren, J. Organomet. Chem. 694, 3215 (2009)
P.S. Chandrachood, A.R. Jadhav, D.R. Garud, N.R. Deshpande, V.G. Puranik, R.V. Kashalkar, Res. Chem. Intermed. 46, 5219 (2020)
J.-T. Hou, Y.-H. Liu, Z.-H. Zhang, J. Heterocycl. Chem. 47, 703 (2010)
P. Vadivel, A. Lalitha, Elixir. Org. Chem. 55, 13013 (2013)
P. Ghosh, A. Mandal, Green Chem. Lett. Rev. 5, 127 (2012)
G. Mohammadi Ziarani, A. Badiei, M. Haddadpour, Int. J. Chem. 3, 87 (2011)
K. Aghapoor, F. Mohsenzadeh, A. Shakeri, H.R. Darabi, M. Ghassemzadeh, B. Neumueller, J. Organomet. Chem. 743, 170 (2013)
P. Renzi, L. Mazzapioda, F. Nardelli, F. Martini, M. Geppi, C. Mancone, M.A. Navarra, F. D’Acunzo, P. Gentili, Eur. J. Inorg. Chem. 2020, 2417 (2020)
Z. Khodaee, A. Yahyazadeh, N.O. Mahmoodi, M.A. Zanjanchi, V. Azimi, J. Mol. Struct. 1029, 92 (2012)
G.C. Nandi, S. Samai, R. Kumar, M.S. Singh, Syn. Commun. 41, 417 (2011)
Y. Zhao, C. Zhang, K.F. Chin, O. Pytela, G. Wei, H. Liu, F. Bureˇs, Z. Jiang, RSC Adv. 4, 30062 (2014)
J. Skalny, J. Maycock, J. Test. Eval. 3, 303 (1975)
S. Papatzani, K. Paine, J. Calabria-Holley, Construct. Build. Mater. 74, 219 (2015)
M.V. Thomas, D.A. Puleo, M. Al-Sabbagh, J. Long-Term, Eff. Med. Implants 15, 599 (2005)
R. Bakhshalinejad, A. Hassanabadi, H. Nassiri-Moghaddam, H. Zarghi, J. Anim. Physiol. Anim. Nutr. 102, 746 (2018)
S.J. Shitole, K.B. Saraf, Cryst. Res. Technol. 37, 440 (2002)
S.J. Shitole, J. Phys. Conf. Ser. 423, 012060 (2013)
M. Parvinzadeh-Gashti, N. Dehghan, J. Solid State Chem. 285, 121262 (2020)
G.E. Ericksen, Geol. Surv. Prof. Pap. (U.S.) 1188, 37 p (1981)
J.C. Oxley, J.L. Smith, M.M. Porter, M.J. Yekel, J.A. Canaria, Explos. Pyrotech. 8, 960 (2017)
H. Wang, J.B. DeLisio, T. Wu, X. Wang, M.R. Zachariah, Powder Technol. 324, 62 (2018)
M. Kalhor, Z. Zarnegar, Res. Chem. Intermed. 48, 519 (2022)
M. Kalhor, Z. Orouji, M. Khalaj, Micropor. Mesopor. Mat. 329, 111498 (2021)
M. Kalhor, S. Banibairami, RSC Adv. 10, 41410 (2020)
S. Brunauer, L.S. Deming, E. Deming, E. Teller, J. Am. Chem. Soc. 62, 1723 (1940)
F. Rouquerol, J. Rouquerol, K.S.W. Sing, Adsorption by powder and porous solids (Academic press, San Diego, 1999), p.1
K.M. El-Shaieb, Heteroat. Chem. 17, 365 (2006)
R. Cristiano, E. Westphal, I.H. Bechtold, A.J. Bortoluzzi, H. Gallardo, Tetrahedron 63, 2851 (2007)
S. Vaghefimoghaddam, H. Valizadeh, J. Iranian Chem. Soc. 13, 1517 (2016)
Acknowledgements
The gratitude of authors goes to the research commute of Chemistry Department of Payame Noor University who provided financial and technical supports for this project.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Definition, supervision, reviewing, editing, initial writing, drawing of figures, and interpretation of nanocatalyst identification analyses were done by MK. The fabrication of nanocatalyst, experiments, and synthesis of the target heterocycles were performed by MS. Writing—review and editing, checking the accuracy of the obtained results, and editing the manuscript were performed by MK. The fabrication of nanocatalyst, experiments, and formal analysis were done by FJ. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
Hereby, I consciously assure that for the manuscript in titled up the following items are fulfilled: The paper is not currently being considered for publication elsewhere. All authors have been personally and actively involved in substantial work leading to the paper and will take public responsibility for its content.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kalhor, M., Shayestefar, M., Khalaj, M. et al. Ca(IO3)2 nanoparticles: fabrication and application as an eco-friendly and recyclable catalyst for the green synthesis of quinoxalines, pyridopyrazines, and 2,3-dicyano pyrazines. Res Chem Intermed 49, 885–900 (2023). https://doi.org/10.1007/s11164-022-04914-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-022-04914-3