Abstract
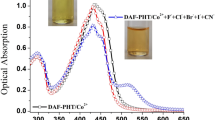
The synthesis of chromeno[d]pyrimidine-2,5-dione/thione derivatives has been described by the reaction of aryl aldehydes, urea/thiourea, and 4-hydroxycoumarin using Fe3O4@SiO2@(BuSO3H)3 as catalyst under microwave irradiation (MWI) in H2O. The sensing application of 4-(4-chlorophenyl)-3,4-dihydro-1H-chromeno[4,3-d]pyrimidine-2,5-dione 4b was examined upon addition of different cations by UV–Vis spectroscopy and exhibited excellent sensitivity towards Hg2+ ions by increasing the absorption. The UV–visible absorption spectral data showed good linearity between the UV–Vis absorption of chemosensor 4b and the concentration of Hg2+ with a detection limit of 3.76 × 10–7 M. Furthermore, the chemosensor 4b exhibited an obvious color change from white to light pink in the presence of Hg2+ in EtOH solution.
Graphic abstract








Similar content being viewed by others
References
M.-O. Simon, C.-J. Li, Chem. Soc. Rev. 41, 1415 (2012)
R.S. Varma, ACS. Sustain. Chem. Eng. 4, 5866 (2016)
G. Mohammadi Ziarani, F. Mohajer, R. Moradi, Green Reactions Under Solvent-Free Conditions, Green Organic Reactions (Springer, Singapore, 2021), pp. 63–83.
R.C. Cioc, E. Ruijter, R.V. Orru, Green Chem. 16, 2958 (2014)
S. Ravichandran, E. Karthikeyan, Int. J. Chem. Tech. Res. 3, 466 (2011)
H.M. Hügel, Molecules 14, 4936 (2009)
J. Govan, Y.K. Gun’ko, Nanomaterials 4, 222 (2014)
L. Järup, Br. Med. Bull. 68, 167 (2003)
A. Renzoni, F. Zino, E. Franchi, Environ. Res. 77, 68 (1998)
P. Kanchana, N. Sudhan, S. Anandhakumar, J. Mathiyarasu, P. Manisankar, C. Sekar, RSC adv. 5, 68587 (2015)
S. Manivannan, Y. Seo, D.-K. Kang, K. Kim, New J. Chem. 42, 20007 (2018)
M.R. Kateshiya, G. George, J.V. Rohit, N.I. Malek, S.K. Kailasa, Microchem. J. 158, 105212 (2020)
M.L. Desai, H. Basu, S. Saha, R.K. Singhal, S.K. Kailasa, J. Mol. Liq. 336, 116239 (2021)
S. Ghosh, J.R. Bhamore, N.I. Malek, Z. Murthy, S.K. Kailasa, Spectrochim. Acta A Mol. Biomol. Spectrosc. 215, 209 (2019)
P. Ju, Z. Wang, Y. Zhang, X. Zhai, F. Jiang, C. Sun, X. Han, Colloids Surf. A: Physicochem. Eng. Asp. 603, 125203 (2020)
H. Ahmad, I.I.B. Sharfan, R.A. Khan, A. Alsalme, Nanomaterials 10, 11 (2020)
B. Amanulla, K.N. Perumal, S.K. Ramaraj, Sens. Actuators, B Chem. 281, 281 (2019)
V.N. Mehta, M.L. Desai, H. Basu, R.K. Singhal, S.K. Kailasa, J. Mol. Liq. 333, 115950 (2021)
Z. Kheilkordi, G. Mohammadi Ziarani, N. Lashgari, A. Badiei, Polyhedron 166, 203 (2019)
M. Darroudi, G. Mohammadi Ziarani, J.B. Ghasemi, A. Badiei, J. Mol. Struct. 1241, 130626 (2021)
Z. Kheilkordi, G. Mohammadi Ziarani, A. Badiei, N. Lashgari, Sensor Lett. 17, 747 (2019)
G. Mohammadi Ziarani, M. Akhgar, F. Mohajer, A. Badiei, R. Luque, 2021. Nanomaterials, 11, 2533 (2021).
G. Mohammadi Ziarani, F. Javadi, F. Mohajer, M. Anafcheh, A. Badiei, J.B. Ghasemi, Mater. Chem. Phys. 275, 125285 (2022).
Z. Kheilkordi, G. Mohammadi Ziarani, S. Bahar, A. Badiei, J. Iran. Chem. Soc. 16, 365 (2019)
G. Mohammadi Ziarani, Z. Ebrahimi, F. Mohajer, A. Badiei, Arab. J. Sci. Eng. (2021). https://doi.org/10.1007/s13369-021-05518-6.
G. Mohammadi Ziarani, M. Akhgar, F. Mohajer, A. Badiei, Res. Chem. Intermed. 47, 2845 (2021)
F. Mohajer, G. Mohammadi Ziarani, A. Badiei, RSC Adv. 11, 6517 (2021)
G. Mohammadi Ziarani, Z. Kheilkordi, F. Mohajer, A. Badiei, R. Luque, RSC Adv. 11, 17456 (2021)
Z. Kheilkordi, G. Mohammadi Ziarani, A. Badiei, Polyhedron 178, 114343 (2020)
H. Mehrabi, M. Baniasad-Dashtabi, J. Chem. Res. 39, 294 (2015)
P.K. Sahu, P.K. Sahu, M.S. Kaurav, M. Messali, S.M. Almutairi, P.L. Sahu, D.D. Agarwal, RSC Adv. 8, 33952 (2018)
A.K. Manna, J. Mondal, R. Chandra, K. Rout, G.K. Patra, J. Photochem. Photobiol. A Chem. 356, 477 (2018)
A.K. Tg, V. Tekuri, M. Mohan, D.R. Trivedi, Sens. Actuators, B Chem. 284, 271 (2019)
H. Hosseinjani-Pirdehi, N.O. Mahmoodi, M.P. Nadamani, A. Taheri, J. Photochem. Photobiol. A Chem. 391, 112365 (2020).
S. Zhang, X. Wu, Q. Niu, Z. Guo, T. Li, H. Liu, J. Fluoresc. 27, 729 (2017)
Acknowledgements
We gratefully acknowledge the Research Council of Alzahra University’s support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jamasbi, N., Mohammadi Ziarani, G., Mohajer, F. et al. A new Hg2+ colorimetric chemosensor: the synthesis of chromeno[d]pyrimidine-2,5-dione/thione derivatives using Fe3O4@SiO2@(BuSO3H)3. Res Chem Intermed 48, 899–909 (2022). https://doi.org/10.1007/s11164-021-04611-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04611-7