Abstract
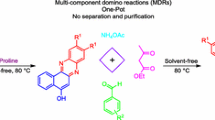
A simple and green synthetic method for preparation of functionalized indol-3-yl pyran derivatives was developed. The reactions were carried out by one-pot three-component synthesis of 3-cyanoacetyl indoles, malononitrile/cyanoacetate and various aldehydes/isatins/acenaphthenequinone in the presence of catalytic amount of l-proline. The advantages of this method included straightforward processing, excellent yields, broad substrate scope and environmental friendliness.






Similar content being viewed by others
References
D. Lee, J.K. Sello, S. Schreiber, Org. Lett. 2, 709 (2000)
I. Macsari, Y. Besidski, G. Csjernyik, L.I. Nilsson, L. Sandberg, U. Yngve, P.I. Arvidsson, J. Med. Chem. 55, 6866 (2012)
D.B. Salunke, E. Yoo, N.M. Shukla, R. Balakrishna, S.S. Malladi, K.J. Serafin, V.W. Day, X. Wang, S.A. David, J. Med. Chem. 55, 8137 (2012)
B. Ganem, Acc. Chem. Res. 42, 463 (2009)
A. Dömling, Chem. Rev. 106, 17 (2006)
A. Dömling, I. Ugi, Angew. Chem. Int. Edit. 39, 3168 (2000)
D.J. Ramón, M. Yus, Angew. Chem. Int. Edit. 44, 1602 (2005)
N. Sultana, P.G. Waterman, Phytochemistry 58, 329 (2001)
T. Kamino, K. Kuramochi, S. Kobayashi, Tetrahedron Lett. 44, 7349 (2003)
D.T. Puerta, J. Mongan, B.L. Tran, J.A. McCammon, C.S.M. Potent, J. Am. Chem. Soc. 127, 14148 (2005)
F. Caturla, M. Amat, R.F. Reinoso, M. Córdoba, G. Warrellow, Bioorg. Med. Chem. Lett. 16, 3209 (2006)
K.I. Nakashima, M. Oyama, T. Ito, J.R. Witono, D. Darnaedi, T. Tanaka, M. Iinuma, Tetrahedron Lett. 52, 4694 (2011)
K.I. Nakashima, M. Oyama, T. Ito, Y. Akao, J.R. Witono, D. Darnaedi, M. Iinuma, Tetrahedron 68, 2421 (2012)
U.C. Rajesh, R. Kholiya, A. Thakur, D.S. Rawat, Tetrahedron Lett. 56, 1790 (2015)
A. Thakur, M. Tripathi, U.C. Rajesh, D.S. Rawat, RSC Adv. 3, 18142 (2013)
R.C. Gadwood, B.V. Kamdar, L.A.C. Dubray, M.L. Wolfe, M.P. Smith, W. Watt, V.E. Groppi, J. Med. Chem. 36, 1480 (1993)
L. Bonsignore, G. Loy, D. Secci, A. Calignano, Eur. J. Med. Chem. 28, 517 (1993)
I.S. Chen, S.J. Wu, I.L. Tsai, T.S. Wu, J.M. Pezzuto, M.C. Lu, C.M. Teng, J. Nat. Prod. 57, 1206 (1994)
K.D. McBrien, Q. Gao, S. Huang, S.E. Klohr, R.R. Wang, D.M. Pirnik, J.E.F. Leet, J. Nat. Prod. 59, 1151 (1996)
Y.L. Yan, S.M. Cohen, Org. Lett. 9, 2517 (2007)
B. Le Bourdonnec, R.T. Windh, L.K. Leister, Q.J. Zhou, C.W. Ajello, M. Gu, R.E. Dolle, J. Med. Chem. 52, 5685 (2009)
B.L. Nilsson, L.E. Overman, J. Org. Chem. 71, 7706 (2006)
D.J. Vugts, M.M. Koningstein, R.F. Schmitz, F.J. de Kanter, M.B. Groen, R.V. Orru, Chem-Eur. J. 12, 7178 (2006)
N.K. Kaushik, N. Kaushik, P. Attri, N. Kumar, C.H. Kim, A.K. Verma, E.H. Choi, Molecules 18, 6620 (2013)
L.H. Franco, E.B.D.K. Joffé, L. Puricelli, M. Tatian, A.M. Seldes, J.A. Palermo, J. Nat. Prod. 61, 1130 (1998)
M.A. Radwan, M. El-Sherbiny, Bioorg. Med. Chem. 15, 1206 (2007)
M.A. Radwan, E.A. Ragab, N.M. Sabry, S.M. El-Shenawy, Bioorg. Med. Chem. 15, 3832 (2007)
M.E. Zaki, M.F. Proença, Tetrahedron 63, 3745 (2007)
P. Wu, Y. Wan, J. Cai, Synlett 8, 1193 (2008)
J.S. Yadav, B.V. Reddy, G. Narasimhulu, N.S. Reddy, P.N. Reddy, K.V. Purnima, B. Jagadeesh, Tetrahedron Lett. 51, 244 (2010)
G.W. Gribble, J. Chem. Soc. Perkin Trans. 1, 1045 (2000)
W.N. Xiong, C.G. Yang, B. Jiang, Bioorg. Med. Chem. 9, 1773 (2001)
K. Parthasarathy, C. Praveen, C. Balachandran, S. Ignacimuthu, P.T. Perumal, Bioorg. Med. Chem. Lett. 23, 2708 (2013)
N.V. Lakshmi, P. Thirumurugan, K.M. Noorulla, P.T. Perumal, Bioorg. Med. Chem. Lett. 20, 5054 (2010)
A. Nandakumar, P. Thirumurugan, P.T. Perumal, P. Vembu, M.N. Ponnuswamy, P. Ramesh, Bioorg. Med. Chem. Lett. 20, 4252 (2010)
T. Chen, X.P. Xu, S.J.J. Ji, Heterocycl. Chem. 50, 244 (2013)
C.L. Shi, D.Q. Shi, S.H. Kim, Z.B. Huang, S.J. Ji, M. Ji, Tetrahedron 64, 2425 (2008)
C.L. Shi, D.Q. Shi, S.H. Kim, Z.B. Huang, M. Ji, Aust. J. Chem. 61, 547 (2008)
C.L. Shi, J.X. Wang, H. Chen, D.Q. Shi, J. Comb. Chem. 12, 430 (2010)
Y.L. Li, H. Chen, C.L. Shi, D.Q. Shi, S.J. Ji, J. Comb. Chem. 12, 231 (2010)
H.Y. Wang, L.L. Li, W. Lin, P. Xu, Z.B. Huang, D.Q. Shi, Org. Lett. 14, 4598 (2012)
B. Alcaide, P. Almendros, A. Luna, M.S. Torres, J. Org. Chem. 71, 4818 (2006)
N. Zotova, A. Franzke, A. Armstrong, D.G. Blackmond, J. Am. Chem. Soc. 129, 15100 (2007)
J.M. Janey, Y. Hsiao, J.D. Armstrong, J. Org. Chem. 71, 390 (2006)
M.L. Kantam, C.V. Rajasekhar, G. Gopikrishna, K.R. Reddy, B.M. Choudary, Tetrahedron Lett. 47, 5965 (2006)
D.B. Ramachary, N.S. Chowdari, C.F. Barbas, Angew. Chem. Int. Edit. 115, 4365 (2003)
M.S. Rasalkar, M.K. Potdar, S.S. Mohile, M.M.J. Salunkhe, Mol. Catal. A Chem. 235, 267 (2005)
A. Kumar, R.A. Maurya, Tetrahedron 2007, 63 (1946)
H. Jiang, R. Mai, H. Cao, Q. Zhu, X. Liu, Org. Biomol. Chem. 7, 4943 (2009)
S. Abdolmohammadi, S. Balalaie, Tetrahedron Lett. 48, 3299 (2007)
S.M. Rajesh, B.D. Bala, S. Perumal, J.C. Menéndez, Green Chem. 13, 3248 (2011)
J.J. Zhang, X. Feng, X.C. Liu, Z.B. Huang, D.Q. Shi, Mol. Divers. 18, 727 (2014)
Li, J.; Wang, J.; Xu, Z.; Zhu, S.L. ACS.Comb.Sci. 2014, 16, 506
S.L. Zhu, S.J. Ji, K. Zhao, Y. Zhang, Y. Liu, Tetrahedron Lett. 49, 2578 (2008)
S.L. Zhu, S.J. Ji, X.M. Su, C. Sun, Y. Liu, Tetrahedron Lett. 49, 1777 (2008)
S.L. Zhu, S.J. Ji, Y. Zhang, Tetrahedron 63, 9365 (2007)
S.L. Zhu, J. Wang, Z. Xu, J. Li, Molecules 17, 13856 (2012)
Acknowledgements
This work was partially supported by Innovation Project of Xuzhou Medical College; Collaborative Innovation Center of Tumor Biological Treatment; Key Laboratory of Organic Synthesis of Jiangsu Province (KJS1010).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jing Wang, Hongzhi Liu and Ren Wen have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Liu, H., Wen, R. et al. l-Proline catalyzed facile and efficient synthesis of functionalized indol-3-yl pyran derivatives by multi-component reactions. Res Chem Intermed 43, 4575–4583 (2017). https://doi.org/10.1007/s11164-017-2897-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-2897-4