Abstract
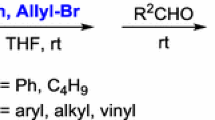
A mild and efficient method for the alkynylation of arylacetylenes with ketones promoted by tert-BuOK under solvent-free conditions was developed. The present green synthesis was applicable to many kinds of aromatic and aliphatic ketones providing good to excellent yields of tertiary propargylic alcohols.
Graphical abstract
A mild and efficient method for the alkynylation of arylacetylenes with ketones promoted by tert-BuOK under solvent-free conditions was developed. The present green synthesis was applicable to many kinds of aromatic and aliphatic ketones providing good to excellent yields of tertiary propargylic alcohols.



Similar content being viewed by others
References
P.T. Anastas, T.C. Williamson, Green Chemistry: Designing Chemistry for the Environment. (ACS symposium series 626, Washington, DC, 1996)
A. Lapkin, D.J.C. Constable, Green Chemistry Metrics: Measuring and Monitoring Sustainable Processes (Wiley-Blackwell, Chichester, 2009)
S.K. Sharma, A. Mudhoo, Green Chemistry for Environmental Sustainability (CRC, Boca Raton, 2011)
P.T. Anastas, L.G. Heine, T.C. Williamson, Green Chemical Syntheses and Processes. (ACS symposium series 767, Washington, DC, 2000)
P. Tundo, V. Esposito, Green Chemical Reactions (Springer, Dordrecht, 2008)
K. Tanaka, Solvent-Free Organic Synthesis, 2nd edn. (Wiley-VCH, Weinheim, 2009)
J.O. Metzger, Angew. Chem. Int. Ed. 37, 2975 (1998)
R.S. Varma, Green Chem. 1, 43 (1999)
K. Tanaka, F. Toda, Chem. Rev. 100, 1025 (2000)
G.W.V. Cave, C.L. Raston, J.L. Scott, Chem. Commun. 2159 (2001)
P.J. Walsh, H. Li, C.A. de Parrodi, Chem. Rev. 107, 2503 (2007)
M.A.P. Martins, C.P. Frizzo, D.N. Moreira, L. Buriol, P. Machado, Chem. Rev. 109, 4140 (2009)
P.G. Cozzi, R. Hilgraf, N. Zimmermann, Eur. J. Org. Chem. 4095 (2004)
P.J. Stang, F. Diederich, Modern Acetylene Chemistry (VCH, Weinheim, 1995)
L. Pu, Tetrahedron 59, 9873 (2003)
B.M. Trost, A.H. Weiss, Adv. Synth. Catal. 351, 963 (2009)
D. Tejedor, S. Lopez-Tosco, F. Cruz-Acosta, G. Mendez-Abt, F. Garcia-Tellado, Angew. Chem. Int. Ed. 48, 2090 (2009)
C.H. Ding, X.L. Hou, Chem. Rev. 111, 1914 (2011)
D.E. Frantz, R. Fassler, C.S. Tomooka, E.M. Carreira, Acc. Chem. Res. 33, 373 (2000)
G. Lu, Y.M. Li, A.S.C. Chan, Coord. Chem. Rev. 249, 1736 (2005)
P.G. Cozzi, R. Hilgraf, N. Zimmermann, Eur. J. Org. Chem. 5969 (2007)
M. Hatano, T. Miyamoto, K. Ishihara, Synthesis 1647 (2008)
K. Tanaka, K. Kukita, T. Ichibakase, S. Kotani, M. Nakajima, Chem. Commun. 5614 (2011)
G.W. Zhang, W. Meng, H. Ma, J. Nie, W.Q. Zhang, J.A. Ma, Angew. Chem. Int. Ed. 50, 3538 (2011)
T. Bauer, S. Smolinski, P. Gawel, J. Jurczak, Tetrahedron Lett. 52, 4882 (2011)
K. Aikawa, Y. Hioki, K. Mikami, Org. Lett. 12, 5713 (2010)
F.Q. Li, S. Zhong, G. Liu, A.S.C. Chan, Adv. Synth. Catal. 351, 1955 (2009)
S. Harada, R. Takita, T. Ohshima, S. Matsunaga, M. Shibasaki, Chem. Commun. 948 (2007)
J. Ekstrom, A.B. Zaitsev, H. Adolfsson, Synlett 885 (2006)
J.H. Babler, V.P. Liptak, N. Phan, J. Org. Chem. 61, 416 (1996)
D. Tzalis, P. Knochel, Angew. Chem. Int. Ed. 38, 1463 (1999)
T. Ishikawa, T. Mizuta, K. Hagiwara, T. Aikawa, T. Kudo, S. Saito, J. Org. Chem. 68, 3702 (2003)
T. Imahori, C. Hori, Y. Kondo, Adv. Synth. Catal. 346, 1090 (2004)
T. Weil, P.R. Schreiner, Eur. J. Org. Chem. 2213 (2005)
H. Miyamoto, S. Yasaka, K. Tanaka, Bull. Chem. Soc. Jpn. 74, 185 (2001)
T.W. Dong, G.W. Wang, L. Wang, Tetrahedron 64, 10148 (2008)
J.F. Liu, J. Lin, L. Song, Tetrahedron Lett. 53, 2160 (2012)
Acknowledgments
The project is generously supported by National Natural Science Foundation of China (Nos. 20902044 and 21102068), Natural Science Foundation of Inner Mongolia of China (No. 2009BS0204) and “Chunhui” Program from Ministry of Education of China (Z2009-1-01003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, S., Yuan, F., Zhao, H. et al. Efficient solvent-free synthesis of tertiary propargylic alcohols from arylacetylenes and ketones promoted by tert-BuOK. Res Chem Intermed 39, 2391–2399 (2013). https://doi.org/10.1007/s11164-012-0765-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0765-9