Abstract
Ultrasound (US) of the thyroid has been used as a diagnostic tool since the late 1960s. US is the most important imaging tool for diagnosing thyroid disease. In the majority of cases a correct diagnosis can already be made in synopsis of the sonographic together with clinical findings and basal thyroid hormone parameters. However, the characterization of thyroid nodules by US remains challenging. The introduction of Thyroid Imaging Reporting and Data Systems (TIRADSs) has improved diagnostic accuracy of thyroid cancer significantly. Newer techniques such as elastography, superb microvascular imaging (SMI), contrast enhanced ultrasound (CEUS) and multiparametric ultrasound (MPUS) expand diagnostic options and tools further. In addition, the use of artificial intelligence (AI) is a promising tool to improve and simplify diagnostics of thyroid nodules and there is evidence that AI can exceed the performance of humans. Combining different US techniques with the introduction of new software, the use of AI, FNB as well as molecular markers might pave the way for a completely new area of diagnostic accuracy in thyroid disease. Finally, interventional ultrasound using US-guided thermal ablation (TA) procedures are increasingly proposed as therapy options for benign as well as malignant thyroid diseases.




Similar content being viewed by others
Data availability
The data that support the findings of this review are openly available at http://doi.org.
Abbreviations
- AI:
-
Artificial intelligence
- ARFI:
-
Acoustic radiation force imaging
- B-mode:
-
Brightness mode
- CAD:
-
Computer-aided diagnosis
- CEUS:
-
Contrast enhanced ultrasound
- CFD:
-
Color flow Doppler
- DL:
-
Deep learning
- EA:
-
Ethanol ablation
- ETA:
-
European Thyroid Association
- FNB:
-
Fine needle biopsy
- FTC:
-
Follicular thyroid carcinoma
- ML:
-
Machine learning
- MPUS:
-
Multiparametric ultrasound
- MTC:
-
Medullary thyroid carcinoma
- NPV:
-
Negative predictive value
- PPV:
-
Positive predictive value
- PRF:
-
Pulse repetition frequency
- PTC:
-
Papillary thyroid carcinoma
- PTMC:
-
Papillary thyroid microcarcinoma
- RFA:
-
Radiofrequency ablation
- SE:
-
Strain elastography
- SMI:
-
Superb microvascular imaging
- SWE:
-
Shear-wave elastography
- TA:
-
Thermal ablation
- THI:
-
Tissue harmonic imaging
- TIC:
-
Time-Intensity-Curve
- TIRADS:
-
Thyroid Imaging Reporting and Data Systems
- US:
-
Ultrasound
- USE:
-
Ultrasound elastography
References
Fujimoto Y, Oka A, Omoto R, et al. Ultrasound scanning of the thyroid gland as a new diagnostic approach. Ultrasonics. 1967;5:177–80. https://doi.org/10.1016/S0041-624X(67)80065-9.
Dighe M, Barr R, Bojunga J, et al. Thyroid ultrasound: State of the art. Part 2 - Focal thyroid lesions. Med Ultrason. 2017;19. https://doi.org/10.11152/mu-999.
Chung J, Lee YJ, Choi YJ, et al. Clinical applications of doppler ultrasonography for thyroid disease: Consensus statement by the korean society of thyroid radiology. Ultrasonography. 2020;39:315–30. https://doi.org/10.14366/usg.20072.
Dighe M, Barr R, Bojunga J, et al. Thyroid ultrasound: State of the art Part 1 - thyroid ultrasound reporting and diffuse thyroid diseases. Med Ultrason. 2017;19:79–93.
Wiest PW, Hartshorne MF, Inskip PD, et al. Thyroid palpation versus high-resolution thyroid ultrasonography in the detection of nodules. J Ultrasound Med. 1998;17:487–96. https://doi.org/10.7863/jum.1998.17.8.487.
Dietrich CF, Müller T, Bojunga J, et al. Statement and recommendations on interventional ultrasound as a thyroid diagnostic and treatment procedure. Ultrasound Med Biol. 2018;44. https://doi.org/10.1016/j.ultrasmedbio.2017.08.1889.
Crocker EF, McLaughlin AF, Kossoff G, et al. The gray scale echographic appearance of thyroid malignancy. J Clin Ultrasound. 1974;2:305–6. https://doi.org/10.1002/JCU.1870020411.
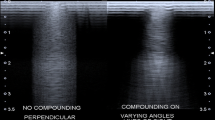
Shapiro RS, Simpson WL, Rausch DL, et al. Compound spatial sonography of the thyroid gland: evaluation of freedom from artifacts and of nodule conspicuity. AJR Am J Roentgenol. 2001;177:1195–8. https://doi.org/10.2214/AJR.177.5.1771195.
Szopinski KT, Wysocki M, Pajk AM, et al. Tissue harmonic imaging of thyroid nodules: initial experience. J Ultrasound Med. 2003;22:5–12. https://doi.org/10.7863/JUM.2003.22.1.5.
Uppal T. Tissue harmonic imaging. Australas J Ultrasound Med. 2010;13:29–31. https://doi.org/10.1002/J.2205-0140.2010.TB00155.X.
Roguin A. Christian Johann Doppler: the man behind the effect. Br J Radiol. 2002;75:615–9. https://doi.org/10.1259/BJR.75.895.750615.
Bamber J, Cosgrove D, Dietrich CF, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013;34:169–84. https://doi.org/10.1055/s-0033-1335205.
Zhao C-K, Xu H-X. Ultrasound elastography of the thyroid: principles and current status. Ultrason (Seoul, Korea). 2019;38:106–24. https://doi.org/10.14366/usg.18037.
Cosgrove D, Barr R, Bojunga J, et al. WFUMB guidelines and recommendations on the clinical use of ultrasound elastography: Part 4. Thyroid. Ultrasound Med Biol. 2016. https://doi.org/10.1016/j.ultrasmedbio.2016.06.022.
Săftoiu A, Gilja OH, Sidhu PS, et al. The EFSUMB guidelines and recommendations for the clinical practice of elastography in non-hepatic applications: Update 2018. Ultraschall der Medizin - Eur J Ultrasound. 2019. https://doi.org/10.1055/a-0838-9937.
Ajmal S. Contrast-enhanced ultrasonography: Review and applications. Cureus. 2021;13:e18243. https://doi.org/10.7759/CUREUS.18243.
Radzina M, Ratniece M, Putrins DS, et al. Performance of contrast-enhanced ultrasound in thyroid nodules: Review of current state and future perspectives. Cancers (Basel). 2021;13:5469. https://doi.org/10.3390/CANCERS13215469.
Trimboli P, Castellana M, Virili C, et al. Performance of contrast-enhanced ultrasound (CEUS) in assessing thyroid nodules: a systematic review and meta-analysis using histological standard of reference. Radiol Medica. 2020;125:406–15. https://doi.org/10.1007/s11547-019-01129-2.
Madariaga AG, Santos Palacios S, Guillén-Grima F, et al. The incidence and prevalence of thyroid dysfunction in Europe: a meta-analysis. J Clin Endocrinol Metab. 2014;99:923–31. https://doi.org/10.1210/JC.2013-2409.
Russ G, Leboulleux S, Leenhardt L, et al. Thyroid incidentalomas: Epidemiology, risk stratification with ultrasound and workup. Eur Thyroid J. 2014;3:154–63. https://doi.org/10.1159/000365289.
Meisinger C, Ittermann T, Wallaschofski H, et al. Geographic variations in the frequency of thyroid disorders and thyroid peroxidase antibodies in persons without former thyroid disease within Germany. Eur J Endocrinol. 2012;167:363–71. https://doi.org/10.1530/EJE-12-0111.
Reiners C, Wegscheider K, Schicha H, et al. Prevalence of thyroid disorders in the working population of Germany: ultrasonography screening in 96,278 unselected employees. Thyroid. 2004;14:926–32. https://doi.org/10.1089/thy.2004.14.926.
Frates MC, Benson CB, Doubilet PM, et al. Prevalence and distribution of carcinoma in patients with solitary and multiple thyroid nodules on sonography. J Clin Endocrinol Metab. 2006;91:3411–7. https://doi.org/10.1210/jc.2006-0690.
Grussendorf M, Ruschenburg I, Brabant G. Malignancy rates in thyroid nodules: a long-term cohort study of 17,592 patients. Eur Thyroid J. 2022;11. https://doi.org/10.1530/ETJ-22-0027.
Haymart MR, Banerjee M, Reyes-Gastelum D, et al. Thyroid ultrasound and the increase in diagnosis of low-risk thyroid cancer. J Clin Endocrinol Metab. 2019;104:785–92. https://doi.org/10.1210/jc.2018-01933.
Lee J-H, Shin SW. Overdiagnosis and screening for thyroid cancer in Korea. Lancet (London, England). 2014;384:1848. https://doi.org/10.1016/S0140-6736(14)62242-X.
Vaccarella S, Franceschi S, Bray F, et al. Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med. 2016;375:614–7. https://doi.org/10.1056/NEJMp1604412.
Ahn HS, Kim HJ, Kim KH, et al. Thyroid cancer screening in South Korea increases detection of papillary cancers with no impact on other subtypes or thyroid cancer mortality. Thyroid. 2016;26:1535–40. https://doi.org/10.1089/thy.2016.0075.
Reinke R, Mathiesen JS, Larsen SR, et al. Incidental and non-incidental papillary thyroid microcarcinoma in Denmark 1996–2015: A national study on incidence, outcome and thoughts on active surveillance. Cancer Epidemiol. 2019;60:46–50. https://doi.org/10.1016/J.CANEP.2019.03.011.
Hofman MS. Thyroid nodules: time to stop over-reporting normal findings and update consensus guidelines. BMJ. 2013;347: f5742.
Cronan JJ. Thyroid nodules: is it time to turn off the US machines? Radiology. 2008;247:602–4. https://doi.org/10.1148/RADIOL.2473072233.
Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for thyroid cancer. JAMA. 2017;317:1882. https://doi.org/10.1001/jama.2017.4011.
Haugen BR, Alexander EK, Bible KC, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016;26:1–133. https://doi.org/10.1089/thy.2015.0020.
Gharib H, Papini E, Garber JR, et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the diagnosis and management of thyroid nodules–2016 update. Endocr Pract. 2016;22. https://doi.org/10.4158/EP161208.GL.
Bojunga J. Ultrasound of thyroid nodules. Ultraschall der Medizin - Eur J Ultrasound. 2018;39:488–511. https://doi.org/10.1055/a-0659-2350.
Remonti LR, Kramer CK, Leitão CB, et al. Thyroid ultrasound features and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid. 2015;25:538–50. https://doi.org/10.1089/thy.2014.0353.
Hoang JK, Middleton WD, Tessler FN. Update on ACR TI-RADS: Successes, challenges, and future directions, from the AJR special series on radiology reporting and data systems. AJR Am J Roentgenol. 2021;216:570–8. https://doi.org/10.2214/AJR.20.24608.
Russ G, Bonnema SJ, Erdogan MF, et al. European thyroid association guidelines for ultrasound malignancy risk stratification of thyroid nodules in adults: The EU-TIRADS. Eur Thyroid J. 2017;6:225–37. https://doi.org/10.1159/000478927.
Shin JH, Baek JH, Chung J, et al. Ultrasonography diagnosis and imaging-based management of thyroid nodules: Revised Korean society of thyroid radiology consensus statement and recommendations. Korean J Radiol. 2016;17:370–95.
Trimboli P, Ferrarazzo G, Deandrea M, et al. Interest of researchers in ultrasound systems for risk stratification of thyroid nodules (TIRADS): a systematic review. Clin Transl Imaging. 2022;10:185–90. https://doi.org/10.1007/s40336-021-00472-7.
Horvath E, Majlis S, Rossi R, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009;94:1748–51. https://doi.org/10.1210/jc.2008-1724.
Trimboli P, Durante C. Ultrasound risk stratification systems for thyroid nodule: between lights and shadows, we are moving towards a new era. Endocrine. 2020;69. https://doi.org/10.1007/s12020-020-02196-6.
Seifert P, Schenke S, Zimny M, et al. Diagnostic performance of Kwak, EU, ACR, and Korean TIRADS as well as ATA guidelines for the ultrasound risk stratification of non-autonomously functioning thyroid nodules in a region with long history of iodine deficiency: A German multicenter trial. Cancers (Basel). 2021;13. https://doi.org/10.3390/CANCERS13174467.
Kim PH, Suh CH, Baek JH, et al. Diagnostic performance of four ultrasound risk stratification systems: A systematic review and meta-analysis. Thyroid. 2020. https://doi.org/10.1089/thy.2019.0812.
Hoang JK, Middleton WD, Farjat AE, et al. Reduction in thyroid nodule biopsies and improved accuracy with American college of radiology thyroid imaging reporting and data system. Radiology. 2018;287:185–93. https://doi.org/10.1148/radiol.2018172572.
Grani G, Lamartina L, Ascoli V, et al. Reducing the number of unnecessary thyroid biopsies while improving diagnostic accuracy: Toward the “Right” TIRADS. J Clin Endocrinol Metab. 2019;104:95–102. https://doi.org/10.1210/jc.2018-01674.
Cosgrove D, Barr R, Bojunga J, et al. WFUMB guidelines and recommendations on the clinical use of ultrasound elastography: Part 4. Thyroid. Ultrasound Med Biol. 2017;43. https://doi.org/10.1016/j.ultrasmedbio.2016.06.022.
Trimboli P, Castellana M, Piccardo A, et al. The ultrasound risk stratification systems for thyroid nodule have been evaluated against papillary carcinoma. A meta-analysis Rev Endocr Metab Disord. 2021;22:453–60. https://doi.org/10.1007/S11154-020-09592-3.
Castellana M, Virili C, Paone G, et al. Ultrasound systems for risk stratification of thyroid nodules prompt inappropriate biopsy in autonomously functioning thyroid nodules. Clin Endocrinol (Oxf). 2020;93:67–75. https://doi.org/10.1111/cen.14204.
Matrone A, Gambale C, Biagini M, et al. Ultrasound features and risk stratification systems to identify medullary thyroid carcinoma. Eur J Endocrinol. 2021;185:193–200. https://doi.org/10.1530/EJE-21-0313.
Ferrarazzo G, Camponovo C, Deandrea M, et al. Suboptimal accuracy of ultrasound and ultrasound-based risk stratification systems in detecting medullary thyroid carcinoma should not be overlooked. Findings from a systematic review with meta-analysis. Clin Endocrinol (Oxf). 2022;97:532–40. https://doi.org/10.1111/CEN.14739.
Trimboli P, Valderrabano P, Pitoia F, et al. Appropriate and mindful measurement of serum calcitonin in patients with thyroid nodules. A white paper Endocrine. 2023. https://doi.org/10.1007/S12020-023-03485-6.
Sugitani I, Ito Y, Takeuchi D, et al. Indications and strategy for active surveillance of adult low-risk papillary thyroid microcarcinoma: Consensus statements from the Japan association of endocrine surgery task force on management for papillary thyroid microcarcinoma. Thyroid. 2021;31:183–92. https://doi.org/10.1089/thy.2020.0330.
Medici M, Liu X, Kwong N, et al. Long- versus short-interval follow-up of cytologically benign thyroid nodules: a prospective cohort study. BMC Med. 2016;14:11. https://doi.org/10.1186/s12916-016-0554-1.
Chou R, Dana T, Mayson SE, et al. Ultrasound follow-up of benign thyroid nodules: A scoping review. Thyroid. 2023;33. https://doi.org/10.1089/THY.2022.0692.
Tessler FN, Thomas J. Artificial intelligence for evaluation of thyroid nodules: a primer. Thyroid. 2023;33:150–8. https://doi.org/10.1089/THY.2022.0560.
Zhao C-K, Ren T-T, Yin Y-F, et al. A comparative analysis of two machine learning-based diagnostic patterns with thyroid imaging reporting and data system for thyroid nodules: Diagnostic performance and unnecessary biopsy rate. Thyroid. 2020. https://doi.org/10.1089/thy.2020.0305.
Wu GG, Lv WZ, Yin R, et al. Deep learning based on ACR TI-RADS can improve the differential diagnosis of thyroid nodules. Front Oncol. 2021;11. https://doi.org/10.3389/FONC.2021.575166.
Peng S, Liu Y, Lv W, et al. Deep learning-based artificial intelligence model to assist thyroid nodule diagnosis and management: a multicentre diagnostic study. Lancet Digit Heal. 2021;3:e250–9. https://doi.org/10.1016/S2589-7500(21)00041-8.
Xu L, Gao J, Wang Q, et al. Computer-aided diagnosis systems in diagnosing malignant thyroid nodules on ultrasonography: a systematic review and meta-analysis. Eur Thyroid J. 2020;9:186–93.
Pacini F, Molinaro E, Castagna MG, et al. Recombinant human thyrotropin-stimulated serum thyroglobulin combined with neck ultrasonography has the highest sensitivity in monitoring differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2003;88:3668–73. https://doi.org/10.1210/jc.2002-021925.
Torlontano M, Crocetti U, Augello G, et al. Comparative evaluation of recombinant human thyrotropin-stimulated thyroglobulin levels, 131I whole-body scintigraphy, and neck ultrasonography in the follow-up of patients with papillary thyroid microcarcinoma who have not undergone radioiodine therapy. J Clin Endocrinol Metab. 2006;91:60–3. https://doi.org/10.1210/jc.2005-1185.
Matrone A, Gambale C, Piaggi P, et al. Postoperative thyroglobulin and neck ultrasound in the risk restratification and decision to perform 131I ablation. J Clin Endocrinol Metab. 2017;102:893–902. https://doi.org/10.1210/jc.2016-2860.
Ko M-S, Lee JH, Shong YK, et al. Normal and abnormal sonographic findings at the thyroidectomy sites in postoperative patients with thyroid malignancy. AJR Am J Roentgenol. 2010;194:1596–609. https://doi.org/10.2214/AJR.09.2513.
Chua WY, Langer JE, Jones LP. Surveillance neck sonography after thyroidectomy for papillary thyroid carcinoma: Pitfalls in the diagnosis of locally recurrent and metastatic disease. J Ultrasound Med. 2017;36:1511–30.
Leenhardt L, Erdogan MF, Hegedus L, et al. 2013 European thyroid association guidelines for cervical ultrasound scan and ultrasound-guided techniques in the postoperative management of patients with thyroid cancer. Eur Thyroid J. 2013;2:147–59. https://doi.org/10.1159/000354537.
Kobaly K, Mandel SJ, Langer JE. Clinical review: Thyroid cancer mimics on surveillance neck sonography. J Clin Endocrinol Metab. 2015;100:371–5.
Shin JH, Han B-K, Ko EY, et al. Sonographic Findings in the Surgical Bed After Thyroidectomy. J Ultrasound Med. 2007;26:1359–66. https://doi.org/10.7863/jum.2007.26.10.1359.
Kamaya A, Gross M, Akatsu H, et al. Recurrence in the thyroidectomy bed: Sonographic findings. Am J Roentgenol. 2011;196:66–70. https://doi.org/10.2214/AJR.10.4474.
Bates MF, Lamas MR, Randle RW, et al. Back so soon? Is early recurrence of papillary thyroid cancer really just persistent disease? Surgery. 2018;163:118–23. https://doi.org/10.1016/j.surg.2017.05.028.
Hong CM, Lee WK, Jeong SY, et al. Superiority of delayed risk stratification in differentiated thyroid cancer after total thyroidectomy and radioactive iodine ablation. Nucl Med Commun. 2014;35:1119–26. https://doi.org/10.1097/MNM.0000000000000183.
Castagna MG, Maino F, Cipri C, et al. Delayed risk stratification, to include the response to initial treatment (surgery and radioiodine ablation), has better outcome predictivity in differentiated thyroid cancer patients. Eur J Endocrinol. 2011;165:441–6. https://doi.org/10.1530/EJE-11-0466.
Ivanac G, Brkljacic B, Ivanac K, et al. Vascularisation of benign and malignant thyroid nodules: CD US evaluation. Ultraschall der Medizin - Eur J Ultrasound. 2007;28:502–6. https://doi.org/10.1055/s-2007-963023.
Brito JP, Gionfriddo MR, Al Nofal A, et al. The accuracy of thyroid nodule ultrasound to predict thyroid cancer: systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99:1253–63. https://doi.org/10.1210/jc.2013-2928.
Moon HJ, Kwak JY, Kim MJ, et al. Can vascularity at power Doppler US help predict thyroid malignancy? Radiology. 2010;255:260–9. https://doi.org/10.1148/radiol.09091284.
Maddaloni E, Briganti SI, Crescenzi A, et al. Usefulness of color doppler ultrasonography in the risk stratification of thyroid nodules. Eur Thyroid J. 2021;10:339–44. https://doi.org/10.1159/000509325.
Rickes S, Sitzy J, Neye H, et al. High-resolution ultrasound in combination with colour-Doppler sonography for preoperative localization of parathyroid adenomas in patients with primary hyperparathyroidism. Ultraschall Med. 2003;24:85–9. https://doi.org/10.1055/s-2003-38667.
MacHado P, Segal S, Lyshchik A, et al. A novel microvascular flow technique: Initial results in thyroids. Ultrasound Q. 2016;32:67–74. https://doi.org/10.1097/RUQ.0000000000000156.
Fu Z, Zhang J, Lu Y, et al. Clinical applications of superb microvascular imaging in the superficial tissues and organs: a systematic review. Acad Radiol. 2021;28:694–703. https://doi.org/10.1016/J.ACRA.2020.03.032.
Lu R, Meng Y, Zhang Y, et al. Superb microvascular imaging (SMI) compared with conventional ultrasound for evaluating thyroid nodules. BMC Med Imaging. 2017;17. https://doi.org/10.1186/S12880-017-0241-5.
Jiang L, Zhang D, Chen Y-N, et al. The value of conventional ultrasound combined with superb microvascular imaging and color Doppler flow imaging in the diagnosis of thyroid malignant nodules: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2023;14. https://doi.org/10.3389/FENDO.2023.1182259.
Friedrich-Rust M, Sperber A, Holzer K, et al. Real-time elastography and contrast-enhanced ultrasound for the assessment of thyroid nodules. Exp Clin Endocrinol Diabetes. 2010;118:602–9. https://doi.org/10.1055/s-0029-1237701.
Friedrich-Rust M, Romenski O, Meyer G, et al. Acoustic Radiation Force Impulse-Imaging for the evaluation of the thyroid gland: A limited patient feasibility study. Ultrasonics. 2012;52. https://doi.org/10.1016/j.ultras.2011.06.012.
Bojunga J, Dauth N, Berner C, et al. Acoustic radiation force impulse imaging for differentiation of thyroid nodules. PLoS One. 2012;7. https://doi.org/10.1371/journal.pone.0042735.
Asteria C, Giovanardi A, Pizzocaro A, et al. US-elastography in the differential diagnosis of benign and malignant thyroid nodules. Thyroid. 2008;18:523–31. https://doi.org/10.1089/thy.2007.0323.
Trimboli P, Guglielmi R, Monti S, et al. Ultrasound sensitivity for thyroid malignancy is increased by real-time elastography: a prospective multicenter study. J Clin Endocrinol Metab. 2012;97:4524–30. https://doi.org/10.1210/jc.2012-2951.
Liu B-J, Li D-D, Xu H-X, et al. Quantitative Shear Wave Velocity Measurement on Acoustic Radiation Force Impulse Elastography for Differential Diagnosis between Benign and Malignant Thyroid Nodules: A Meta-analysis. Ultrasound Med Biol. 2015;41:3035–43. https://doi.org/10.1016/j.ultrasmedbio.2015.08.003.
Zhan J, Jin J-M, Diao X-H, et al. Acoustic radiation force impulse imaging (ARFI) for differentiation of benign and malignant thyroid nodules-A meta-analysis. Eur J Radiol. 2015;84:2181–6. https://doi.org/10.1016/j.ejrad.2015.07.015.
Bojunga J, Herrmann E, Meyer G, et al. Real-time elastography for the differentiation of benign and malignant thyroid nodules: A meta-analysis. Thyroid. 2010;20. https://doi.org/10.1089/thy.2010.0079.
Maxim LD, Niebo R, Utell MJ. Screening tests: a review with examples. Inhal Toxicol. 2014;26:811–28. https://doi.org/10.3109/08958378.2014.955932.
Friedrich-Rust M, Vorlaender C, Dietrich CF, et al. Evaluation of strain elastography for differentiation of thyroid nodules: Results of a prospective DEGUM multicenter study. Ultraschall Med. 2016;37:262–70. https://doi.org/10.1055/s-0042-104647.
Nell S, Kist JW, Debray TPA, et al. Qualitative elastography can replace thyroid nodule fine-needle aspiration in patients with soft thyroid nodules. A systematic review and meta-analysis. Eur J Radiol. 2015;84:652–61. https://doi.org/10.1016/j.ejrad.2015.01.003.
Vorländer C, Wolff J, Saalabian S, et al. Real-time ultrasound elastography–a noninvasive diagnostic procedure for evaluating dominant thyroid nodules. Langenbeck’s Arch Surg/Dtsch Gesellschaft für Chir. 2010;395:865–71. https://doi.org/10.1007/s00423-010-0685-3.
Bhatia KSS, Rasalkar DP, Lee YP, et al. Cystic change in thyroid nodules: a confounding factor for real-time qualitative thyroid ultrasound elastography. Clin Radiol. 2011;66:799–807. https://doi.org/10.1016/j.crad.2011.03.011.
Cantisani V, De Silvestri A, Scotti V, et al. US-elastography with different techniques for thyroid nodule characterization: Systematic review and meta-analysis. Front Oncol. 2022;12. https://doi.org/10.3389/FONC.2022.845549.
Andrioli M, Trimboli P, Amendola S, et al. Elastographic presentation of medullary thyroid carcinoma. Endocrine. 2014;45:153–5. https://doi.org/10.1007/S12020-013-0062-4.
Cantisani V, Maceroni P, D’Andrea V, et al. Strain ratio ultrasound elastography increases the accuracy of colour-Doppler ultrasound in the evaluation of Thy-3 nodules. A bi-centre university experience Eur Radiol. 2015. https://doi.org/10.1007/s00330-015-3956-0.
Chen L, Shi Y-X, Liu Y-C, et al. The values of shear wave elastography in avoiding repeat fine-needle aspiration for thyroid nodules with nondiagnostic and undetermined cytology. Clin Endocrinol (Oxf). 2019;91:201–8. https://doi.org/10.1111/cen.13992.
Ruhlmann M, Stebner V, Görges R, et al. Diagnosis of hyperfunctional thyroid nodules: impact of US-elastography. Nuklearmedizin. 2014;53:173–7. https://doi.org/10.3413/NUKMED-0660-14-04.
Trimboli P, Paone G, Zatelli MC, et al. Real-time elastography in autonomously functioning thyroid nodules: relationship with TSH levels, scintigraphy, and ultrasound patterns. Endocrine. 2017;58:488–94. https://doi.org/10.1007/s12020-017-1277-6.
Yang Z, Zhang H, Wang K, et al. Assessment of diffuse thyroid disease by strain ratio in ultrasound elastography. Ultrasound Med Biol. 2015;41:2884–9. https://doi.org/10.1016/j.ultrasmedbio.2015.07.012.
Menzilcioglu MS, Duymus M, Gungor G, et al. The value of realtime ultrasound elastography in chronic autoimmune thyroiditis. Br J Radiol. 2014;87. https://doi.org/10.1259/bjr.20140604.
Cepeha CM, Paul C, Borlea A, et al. The value of strain elastography in predicting autoimmune thyroiditis. Diagnostics (Basel, Switzerland). 2020;10. https://doi.org/10.3390/DIAGNOSTICS10110874.
Li X, Gao F, Li F, et al. Qualitative analysis of contrast-enhanced ultrasound in the diagnosis of small, TR3-5 benign and malignant thyroid nodules measuring ≤1 cm. Br J Radiol. 2020;93:20190923. https://doi.org/10.1259/bjr.20190923.
Liu Q, Cheng J, Li J, et al. The diagnostic accuracy of contrast-enhanced ultrasound for the differentiation of benign and malignant thyroid nodules A PRISMA compliant meta-analysis. Med (United States) 2018;97.
Xu Y, Qi X, Zhao X, et al. Clinical diagnostic value of contrast-enhanced ultrasound and ti-rads classification for benign and malignant thyroid tumors one comparative cohort study. Med (United States). 2019;98. https://doi.org/10.1097/MD.0000000000014051.
Xi X, Gao L, Wu Q, et al. Differentiation of thyroid nodules difficult to diagnose with contrast-enhanced ultrasonography and real-time elastography. Front Oncol. 2020;10. https://doi.org/10.3389/fonc.2020.00112.
Agha A, Hornung M, Stroszczynski C, et al. Highly efficient localization of pathological glands in primary hyperparathyroidism using contrast-enhanced ultrasonography (CEUS) in comparison with conventional ultrasonography. J Clin Endocrinol Metab. 2013;98:2019–25. https://doi.org/10.1210/jc.2013-1007.
Hu C, Feng Y, Huang P, et al. Adverse reactions after the use of SonoVue contrast agent: Characteristics and nursing care experience. Medicine (Baltimore). 2019;98: e17745. https://doi.org/10.1097/MD.0000000000017745.
Cantisani V, D’Ambrosio F, Nielsen MB. Multiparametric ultrasound of thyroid nodules: Where do we stand? Ultraschall Med. 2017;38:357–9. https://doi.org/10.1055/S-0043-111682.
Brandenstein M, Wiesinger I, Künzel J, et al. Multiparametric sonographic imaging of thyroid lesions: Chances of B-mode, elastography and CEUS in relation to preoperative histopathology. Cancers (Basel). 2022;14:4745. https://doi.org/10.3390/CANCERS14194745.
Fresilli D, David E, Pacini P, et al. Thyroid nodule characterization: how to assess the malignancy risk. Update of the literature. Diagnostics (Basel, Switzerland). 2021;11. https://doi.org/10.3390/DIAGNOSTICS11081374.
Hahn SY, Shin JH, Na DG, et al. Ethanol ablation of the thyroid nodules: 2018 consensus statement by the Korean society of thyroid radiology. Korean J Radiol. 2019;20:609–20. https://doi.org/10.3348/kjr.2018.0696.
Deandrea M, Trimboli P, Creanza A, et al. Long-term follow-up of cystic thyroid nodules treated with percutaneous ethanol injection (PEI) using two different approaches. Eur J Endocrinol. 2020;183:489–95. https://doi.org/10.1530/EJE-20-0213.
Gong X, Zhou Q, Chen S, et al. Efficacy and safety of ultrasound-guided percutaneous polidocanol sclerotherapy in benign predominantly cystic thyroid nodules: a prospective study. Curr Med Res Opin. 2017;33:1505–10. https://doi.org/10.1080/03007995.2017.1325732.
Gong X, Wang F, Du H, et al. Comparison of ultrasound-guided percutaneous polidocanol injection versus percutaneous ethanol injection for treatment of benign cystic thyroid nodules. J Ultrasound Med. 2018;37:1423–9. https://doi.org/10.1002/jum.14482.
Na DG, Lee JH, Jung SL, et al. Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol. 2012;13:117–25. https://doi.org/10.3348/KJR.2012.13.2.117.
Papini E, Pacella CM, Solbiati LA, et al. Minimally-invasive treatments for benign thyroid nodules: a Delphi-based consensus statement from the Italian minimally-invasive treatments of the thyroid (MITT) group. Int J Hyperth. 2019;36:376–82. https://doi.org/10.1080/02656736.2019.1575482.
Feldkamp J, Grünwald F, Luster M, et al. Non-surgical and non-radioiodine techniques for ablation of benign thyroid nodules: Consensus statement and recommendation. Exp Clin Endocrinol Diabetes. 2020. https://doi.org/10.1055/a-1075-2025.
Papini E, Monpeyssen H, Frasoldati A, et al. 2020 European thyroid association clinical practice guideline for the use of image-guided ablation in benign thyroid nodules. Eur Thyroid J. 2020;9:172–85. https://doi.org/10.1159/000508484.
Trimboli P, Castellana M, Sconfienza LM, et al. Efficacy of thermal ablation in benign non-functioning solid thyroid nodule: A systematic review and meta-analysis. Endocrine. 2020;67:35–43. https://doi.org/10.1007/S12020-019-02019-3.
Chung SR, Baek JH, Choi YJ, et al. Thermal ablation for the management of papillary thyroid microcarcinoma in the era of active surveillance and hemithyroidectomy. Curr Oncol Rep. 2022;24:1045–52. https://doi.org/10.1007/s11912-022-01268-2.
Miyauchi A, Ito Y, Fujishima M, et al. Long-term outcomes of active surveillance and immediate surgery for adult patients with low-risk papillary thyroid microcarcinoma: 30-year experience. Thyroid. 2023;33. https://doi.org/10.1089/THY.2023.0076.
Cho SJ, Baek SM, Na DG, et al. Five-year follow-up results of thermal ablation for low-risk papillary thyroid microcarcinomas: systematic review and meta-analysis. Eur Radiol. 2021;31:6446–56. https://doi.org/10.1007/S00330-021-07808-X.
Chen Z, Zhang W, He W. Ultrasound-guided thermal ablation for papillary thyroid microcarcinoma: A systematic review. Clin Endocrinol (Oxf). 2023;98:296–305. https://doi.org/10.1111/CEN.14857.
Yan L, Yang Z, Li Y, et al. Five-year outcome between radiofrequency ablation vs. surgery for unilateral multifocal papillary thyroid microcarcinoma. J Clin Endocrinol Metab. 2023. https://doi.org/10.1210/CLINEM/DGAD360.
Funding
None.
Author information
Authors and Affiliations
Contributions
JB literature search, writing and editing of the manuscript; PT writing and editing of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
N/A.
Informed consent
N/A.
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bojunga, J., Trimboli, P. Thyroid ultrasound and its ancillary techniques. Rev Endocr Metab Disord 25, 161–173 (2024). https://doi.org/10.1007/s11154-023-09841-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-023-09841-1