Abstract
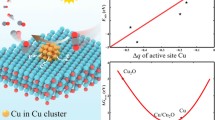
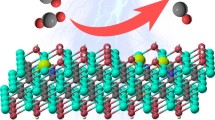
This work proposes the activated mechanical alloyed composite catalysts by in-situ O2 heat treatment (CuxSn100−xO2HT). The catalytic activity of catalysts is improved tremendously for the phenol hydroxylation by superoxide (O2−). The conversion is almost 100% and selective to 94% catechol (CAT). To understand the formation of superoxide, in situ time-resolved Cu K-edge and Sn L3-edge X-ray absorption spectroscopy explain the contribution of an oxygen atom to the CuxSny composites. Meanwhile, in-situ XAS with O2 heat treatment depicts how superoxide bonded on CuxSny composites. Quantitatively, H2-TPR and curve fitting ATR-FTIR determine the total amount of superoxide on MAed CuxSn100−xO2HT and distinguish the numbers of O2− on Cu and Sn sites in CuxSn100−xO2HT. EPR explains that the hybridization of d-orbitals of Cu2+ and p-d orbitals of Sn4+ shifted the g-factor of a free electron in superoxide. XPS depth profiling of O 1s reveals identical superoxide on the composite CuxSnyO2HT catalysts. After optimizing the Sn contents, Cu30Sn70O2HT exhibits the highest catalytic activity and a number of superoxides, giving the CAT 183 μmol in conjunction with reducing tar contents. Extended X-ray absorption fine structure (EXAFS) illustrates the present compressive strain of Cu–O distance and is essential in increasing the quantity of CAT. The work presents how mechanical alloying can be a potential free-solvent catalyst synthesis.










Similar content being viewed by others
References
Costa JAS, de Jesus RA, Santos DO, Neris JB, Figueiredo RT, Paranhos CM (2021) Synthesis, functionalization, and environmental application of silica-based mesoporous materials of the M41S and SBA-n families: a review. J Environ Chem Eng 9:105259. https://doi.org/10.1016/j.jece.2021.105259
Xia K, Lang W-Z, Li P-P, Yan X, Guo Y-J (2016) The properties and catalytic performance of PtIn/Mg(Al)O catalysts for the propane dehydrogenation reaction: effects of pH value in preparing Mg(Al)O supports by the co-precipitation method. J Catal 338:104–114. https://doi.org/10.1016/j.jcat.2016.02.028
Ahmadi R, Amini MK, Bennett JC (2012) Pt–Co alloy nanoparticles synthesized on sulfur-modified carbon nanotubes as electrocatalysts for methanol electrooxidation reaction. J Catal 292:81–89. https://doi.org/10.1016/j.jcat.2012.05.001
del Río E, Gaona D, Hernández-Garrido JC, Calvino JJ, Basallote MG, Fernández-Trujillo MJ, Pérez-Omil JA, Gatica JM (2014) Speciation-controlled incipient wetness impregnation: a rational synthetic approach to prepare sub-nanosized and highly active ceria–zirconia supported gold catalysts. J Catal 318:119–127. https://doi.org/10.1016/j.jcat.2014.07.001
Adabavazeh Z, Karimzadeh F, Enayati MH (2012) Synthesis and structural characterization of nanocrystalline (Ni, Fe)3Al composite compound prepared by mechanical alloying. Adv Powder Technol 23:284–289. https://doi.org/10.1016/j.apt.2011.03.012
Borchers C, Garve C, Tiegel M, Deutges M, Herz A, Edalati K, Pippan R, Horita Z, Kirchheim R (2015) Nanocrystalline steel obtained by mechanical alloying of iron and graphite subsequently compacted by high-pressure torsion. Acta Mater 97:207–215. https://doi.org/10.1016/j.actamat.2015.06.049
Besson R, Avettand-Fènoël MN, Thuinet L, Kwon J, Addad A, Roussel P, Legris A (2015) Mechanisms of formation of Al4Cu9 during mechanical alloying: an experimental study. Acta Mater 87:216–224. https://doi.org/10.1016/j.actamat.2014.12.050
Pasebani S, Charit I, Wu YQ, Butt DP, Cole JI (2013) Mechanical alloying of lanthana-bearing nanostructured ferritic steels. Acta Mater 61:5605–5617. https://doi.org/10.1016/j.actamat.2013.06.002
Pithakratanayothin S, Tongsri R, Wongkasemjit S, Chaisuwan T (2016) A simple route to CuxSn(100–x) composite nanoparticle catalyst for ultra-phenol hydroxylation. Mat Chem Phys 181:452–461. https://doi.org/10.1016/j.matchemphys.2016.06.081
Klaewkla R, Kulprathipanja S, Rangsunvigit P, Rirksomboon T, Rathbun W, Nemeth L (2007) Kinetic modelling of phenol hydroxylation using titanium and tin silicalite-1s: effect of tin incorporation. Chem Eng J 129:21–30. https://doi.org/10.1016/j.cej.2006.10.034
Vu BK, Song MB, Ahn IY, Suh Y-W, Suh DJ, Kim W-I, Koh H-L, Choi YG, Shin EW (2011) Pt–Sn alloy phases and coke mobility over Pt–Sn/Al2O3 and Pt–Sn/ZnAl2O4 catalysts for propane dehydrogenation. Appl Catal A 400:25–33. https://doi.org/10.1016/j.apcata.2011.03.057
Gianotti E, Reyes-Carmona Á, Taillades-Jacquin M, Taillades G, Rozière J (2014) Study of the effect of addition of Into Pt-Sn/γ-Al2O3 catalysts for high purity hydrogen production via partial dehydrogenation of kerosene jet A-1. Appl Catal B 160–161:574–581. https://doi.org/10.1016/j.apcatb.2014.06.003
Fürtauer S, Li D, Cupid D, Flandorfer H (2013) The Cu–Sn phase diagram, part I: new experimental results. Composites 34:142–147. https://doi.org/10.1016/j.intermet.2012.10.004
Pan Y, Xu X, Zhong Y, Ge L, Chen Y, Veder JM, Guan D, O’Hayre R, Li M, Wang G, Wang H, Zhou W, Shao Z (2020) Direct evidence of boosted oxygen evolution over perovskite by enhanced lattice oxygen participation. Nat Commun 11:2002. https://doi.org/10.1038/s41467-020-15873-x
Yoo JS, Rong X, Liu Y, Kolpak AM (2018) Role of lattice oxygen participation in understanding trends in the oxygen evolution reaction on perovskites. ACS Catal 8:4628–4636. https://doi.org/10.1021/acscatal.8b00612
Liu D, Ai H, Li J, Fang M, Chen M, Liu D, Du X, Zhou P, Li F, Lo KH, Tang Y, Chen S, Wang L, Xing G, Pan H (2020) Surface reconstruction and phase transition on vanadium–cobalt–iron trimetal nitrides to form active oxyhydroxide for enhanced electrocatalytic water oxidation. Adv Energy Mater 10:2002464. https://doi.org/10.1002/aenm.202002464
Anand R, Nissimagoudar AS, Umer M, Ha M, Zafari M, Umer S, Lee G, Kim KS (2021) Late transition metal doped MXenes showing superb bifunctional electrocatalytic activities for water splitting via distinctive mechanistic pathways. Energy Mater Adv. https://doi.org/10.1002/aenm.202102388
Harzandi AM, Shadman S, Nissimagoudar AS, Kim DY, Lim HD, Lee JH, Kim MG, Jeong HY, Kimand Y, Kim KS (2021) Ruthenium core-shell engineering with nickel single atoms for selective oxygen evolution via nondestructive mechanism. Adv Energy Mater 11:2003448. https://doi.org/10.1002/aenm.202003448
Guan D, Zhang K, Hu Z, Wu X, Chen JL, Pao CW, Guo Y, Zhou W, Shao Z (2021) Exceptionally robust face-sharing motifs enable efficient and durable water oxidation. Adv Mater 33:2103392. https://doi.org/10.1002/adma.202103392
Wang C, Zhai P, Xia M, Wu Y, Zhang B, Li Z, Ran L, Gao J, Zhang X, Fan Z (2021) Engineering lattice oxygen activation of iridium clusters stabilized on amorphous bimetal borides array for oxygen evolution reaction. Angew Chem Int Ed 60:27126–27134. https://doi.org/10.1002/anie.202112870
Cullity BD (2001) Elements of X-ray diffraction, 2nd edn. Addison-Wesley Publishing Company Inc, Boston
Callister WD (2003) Materials science and engineering: an introduction. Wiley, New York
Howard SA, Preston KD (1989) Modern powder diffraction. The Mineralogical Society of America, Washington 20:217–275
Buarod E, Pithakratanayothin S, Naknaka S, Chaiyasith P, Yotkaew T, Tosangthum N, Tongsri R (2015) Facile synthesis and characterization of tenorite nanoparticles from gas-atomized Cu powder. Powder Technol 269:118–126. https://doi.org/10.1016/j.powtec.2014.08.052
Jia W, Zhang J, Zuo M, Yu X, Liu H, Li Z, Sun Y, Yang S, Tang X, Zeng X, Lin L (2022) Facile one-pot synthesis of furan double Schiff base from 5-hydroxymethylfurfural via an amination–oxidation–amination strategy in water. ACS Sustain Chem Eng 10:421–430. https://doi.org/10.1021/acssuschemeng.2c01576
Baronetti GT, de Miguel SR, Scelza OA, Castro AA (1986) State of metallic phase in PtSn/Al2O3 catalysts prepared by different deposition techniques. Appl Catal 24:109–116. https://doi.org/10.1016/j.catcom.2006.01.027
Chetri P, Choudhury B, Choudhury A (2014) Room temperature ferromagnetism in SnO2 nanoparticles: an experimental and density functional study. J Mater Chem C 2:9294–9302. https://doi.org/10.1039/C4TC01070A
Che M, Tench AJ (1983) Characterization and reactivity of molecular oxygen species on oxide surfaces. Adv Catal 32:1–148. https://doi.org/10.1016/S0360-0564(08)60439-3
Xue J, Wang X, Qi G, Wang J, Shen M, Li W (2013) Characterization of copper species over Cu/SAPO-34 in selective catalytic reduction of NOx with ammonia: Relationships between active Cu sites and de-NOx performance at low temperature. J Catal 297:56–64. https://doi.org/10.1016/j.jcat.2012.09.020
Martínez-arias A, Conesa JC, Soria J (2007) O2-probe EPR as a method for characterization of surface oxygen vacancies in ceria-based catalysts. Res Chem Intermed 33:775–791. https://doi.org/10.1163/156856707782169345
Soria J, Martínez-Arias A, Conesa JC (1995) Spectroscopic study of oxygen adsorption as a method to study surface defects on CeO2. J Chem Soc Faraday Trans 91:1669. https://doi.org/10.1039/FT9959101669
Martínez-Arias A, Fernández-García M, Salamanca LN, Valenzuela RX, Conesa JC, Soria J (2000) Structural and redox properties of ceria in alumina-supported ceria catalyst supports. J Phys Chem B 104:4038. https://doi.org/10.1021/jp992796y
Niu X, Zhao T, Yuan F, Zhu Y (2014) Preparation of Hollow CuO@SiO2 spheres and its catalytic performances for the NO + CO and CO oxidation. Sci Rep 5:9153. https://doi.org/10.1038/srep09153
Liu Z, Handa K, Kaibuchi K, Tanaka Y, Kawai J (2004) Comparison of the Sn L edge X-ray absorption spectra and the corresponding electronic structure in Sn, SnO, and SnO2. J Electron Spectrosc Relat Phenomena 135:155–160
Koningsberger DC, Prins R (1988) X-ray absorption: principles, applications, techniques of EXAFS, SEXAFS, and XANES. Wiley, New York, p 673
Prayoonphokkharat P, Amonpattarakit P, Watchrapasorn A (2020) Crystal structure and XANES study of Sn-substituted YBa2Cu3O7-y powder prepared by solid-state synthesis method. Appl Phys A. https://doi.org/10.1007/s00339-020-3330-1
Groothaert MH, van Bokhoven JA, Battiston AA, Weckhuysen BM, Schoonheydt RA (2003) Selective oxidation of methane by the bis(μ-oxo) dicopper core stabilized on ZSM-5 and mordenite zeolites. J Am Chem Soc 125(2003):7629–7640. https://doi.org/10.1021/ja047158u
Cheng N, Wei YL, Yang YW, Lee JF (2005) Effect of water on XAS spectrum of Cu(NO3)2 reference. Phys Scr 115:907–908. https://doi.org/10.1238/physica.topical.115a00907
Chaboy J, Munoz-Paez A, Carrera F, Merkling P, Marcos ES (2005) Ab initio x-ray absorption study of copper K-edge XANES spectra in Cu(II) compounds. Phys Rev B 71:134208. https://doi.org/10.1103/PhysRevB.71.134208
Lieberman RL, Rosenzweig AC (2005) Crystal structure of a membrane-bound metalloenzyme that catalyzes the biological oxidation of methane. Nature 434:177–182
Balasubramanian R, Smith SM, Rawat S, Yatsunyk LA, Stemmler TL, Rosenzweig AC (2010) Oxidation of methane by a biological dicopper centre. Nature 465:115–131. https://doi.org/10.1038/nature08992
Sushkevich VL, Palagin D, Ranocchiari M, van Bokhoven JA (2017) Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 356:523–527. https://doi.org/10.1126/science.aam9035
Vanelderen P, Snyder BER, Tsai M, Hadt RG, Vancauwenbergh J, Coussens O, Schoonheydt RA, Sels BF, Solmon EI (2015) Spectroscopic definition of the copper active sites in mordenite: selective methane oxidation. J Am Chem Soc 137:6383–6392. https://doi.org/10.1021/jacs.5b02817
Oswald HR, Reller A, Schmalle HW, Dubler E (1990) Structure of copper(II) hydroxide, Cu(OH)2. Acta Crystallogr Sect C 46:2279–2284. https://doi.org/10.1107/S0108270190006230
Forsyth JB, Hull S (1991) The effect of hydrostatic pressure on the ambient temperature structure of CuO. J Phys 3:5257. https://doi.org/10.1088/0953-8984/3/28/001
LoJacono M, Fierro G, Dragone R, FengDitri J, Hall WK XB (1997) Zeolite chemistry of CuZSM-5 revisited. J Phys Chem B 101:1979–1984. https://doi.org/10.1021/jp9632044
Takao G, Hiroya O, Yu I, Yubin L, Fumihiro N, Masahiro M, Futoshi M (2019) Electrocatalytic conversion of carbon dioxide to formic acid over nanosized Cu6Sn5 composite compounds with a SnO2 shell layer. Catal Sci Technol 23(23):6577–6584. https://doi.org/10.1039/C9CY01540J
Neylon MK, Marshall CL, Kropf AJ (2002) In situ EXAFS analysis of the temperature-programmed reduction of Cu-ZSM-5. J Am Chem Soc 124:5457–5465. https://doi.org/10.1021/ja0176696
Cheng W, Zhao X, Su H (2019) Lattice-strained metal–organic-framework arrays for bifunctional oxygen electrocatalysis. Nat Energy 4:115–122. https://doi.org/10.1038/s41560-018-0308-8
Xiong Y, Yao X, Tang C, Zhang L, Cao Y, Deng Y, Gao F, Dong L (2014) Effect of CO-pretreatment on the CuO–V2O5/γ-Al2O3 catalyst for NO reduction by CO. Catal Sci Technol 4:4416–4425. https://doi.org/10.1039/C4CY00785A
Chanapattharapol KC, Krachuamram S, Kidkhunthod P, Poo-arporn Y (2020) The effect of Sm addition on structure, redox properties and catalytic activities for water gas shift reaction of ceria-based support. Solid State Sci 99:106066. https://doi.org/10.1016/j.solidstatesciences.2019.106066
Acknowledgements
The authors gratefully acknowledge the mutual support or partial scholarship from The Petroleum and Petrochemical college; Ratchadaphiseksompote Endowment Fund, Chulalongkorn University; Thailand Research Fund (Senior Research Scholar); and Powder Metallurgy Research and Development Unit (PM_RDU) of the National Metal and Materials Technology Center (MTEC). The authors also would like to thank Dr. Robert Butcher for proofreading the paper.
Funding
Funding was provided by National Metal and Materials Technology Center and Petroleum and Petrochemical College, Chulalongkorn University.
Author information
Authors and Affiliations
Contributions
SP: Conceptualization; methodology; validation; writing—original draft; writing—review and editing, supervision, project administration. SN: Writing—original draft, visualization, methodology, software. RT: Funding acquisition. TC: Validation, funding acquisition; writing—review and editing. PK: Funding acquisition. SW: Validation, funding acquisition; writing—review and editing. DB: Visualization. EB: Visualization, validation, investigation.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pithakratanayothin, S., Numbenjapon, S., Tongsri, R. et al. In-situ X-ray absorption spectroscopy study on the formation of superoxides on CuxSny composite catalysts enables the direct synthesis of catechol. Reac Kinet Mech Cat 136, 3079–3104 (2023). https://doi.org/10.1007/s11144-023-02518-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02518-5