Abstract
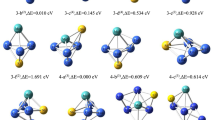
Density functional theory (DFT) is used to investigate the N2O decomposition over Pd4−/0/+ clusters. The Eley–Rideal (ER) mechanism and the Langmuir–Hinshelwood (LH) mechanism are well established. The average binding energies show that the most stable structure of Pd4−/0/+ clusters is the tetrahedral configuration. For the Pd4− cluster, the activation energies indicate that the rate-limiting step in two mechanisms is the formation of O2, and the ER mechanism occurs more easily than the LH mechanism. While for the Pd40 and Pd4+ clusters, the rate-limiting step in two mechanisms is the N2O decomposition to N2, and the LH mechanism is more likely to process. Among all clusters, the Pd4− cluster exhibits better catalytic activity compared with the Pd40 and Pd4+ clusters.





Similar content being viewed by others
References
Itokawa H, Hanaki K, Matsuo T (2001) Nitrous oxide production in high-loading biological nitrogen removal process under low cod/n ratio condition. Water Res 35(3):657–664. https://doi.org/10.1016/S0043-1354(00)00309-2
Yan X, Zheng SK, Qiu DZ, Yang J, Han YP, Huo ZM, Su XF, Sun JH (2019) Characteristics of N2O generation within the internal micro-environment of activated sludge flocs under different dissolved oxygen concentrations. Bioresour Technol 291:121867. https://doi.org/10.1016/j.biortech.2019.121867
Tian HQ et al (2020) A comprehensive quantification of global nitrous oxide sources and sinks. Nature 586:248–256. https://doi.org/10.1038/s41586-020-2780-0
Shakoor A et al (2021) Effect of animal manure, crop type, climate zone, and soil attributes on greenhouse gas emissions from agricultural soils-A global meta-analysis. J Clean Prod 278:124019. https://doi.org/10.1016/j.jclepro.2020.124019
Tanaka S, Yuzaki K, Ito S, Kameoka S, Kunimori K (2001) Mechanism of O2 desorption during N2O decomposition on an oxidized Rh/USY catalyst. J Catal 200(2):203–208. https://doi.org/10.1006/jcat.2001.3197
Yamashita T, Vannice A (1996) N2O decomposition over manganese oxides. J Catal 161(1):254–262. https://doi.org/10.1006/jcat.1996.0183
Francisco H, Bertin V, Soto JR, Castro M (2016) Charge and geometrical effects on the catalytic N2O reduction by Rh6- and Rh6+ clusters. J Phys Chem C 120(41):23648–23659. https://doi.org/10.1021/acs.jpcc.6b08172
Wu LY, Chen C, Luo L, Wang YC, Yin B (2020) DFT Study of the reaction mechanism of N2O decomposition on Au3+/0/- clusters. ChemistrySelect 5:5391–5399. https://doi.org/10.1002/slct.202000752
Lian X, Guo WL, Nie Y, Xu P, Yi H, He B, Chen SK (2019) A density functional study of water dissociation on small cationic, neutral, and anionic Ni-based alloy clusters. Chem Phys 521:44–50. https://doi.org/10.1016/j.chemphys.2019.01.019
Omrani M, Goriaux M, Liu Y, Martinet S, Jean-Soro L, Ruban V (2020) Platinum group elements study in automobile catalysts and exhaust gas samples. Environ Pollut 257:113477. https://doi.org/10.1016/j.envpol.2019.113477
Cao YD, Ran R, Wu XD, Si ZC, Kang FY, Weng D (2022) Progress on metal-support interactions in Pd-based catalysts for automobile emission control. J Environ Sci 125:401–426. https://doi.org/10.1016/j.jes.2022.01.011
Kim K, Baek S, Kim JJ, Han JW (2020) Catalytic decomposition of N2O on PdxCuy alloy catalysts: a density functional theory study. Appl Surf Sci 510:145349. https://doi.org/10.1016/j.apsusc.2020.145349
Xing W, Yang XF, Wang AQ, Li L, Liu XY, Zhang T, Mou CY, Li J (2012) Bimetallic Au-Pd alloy catalysts for N2O dissociation: effects of surface structures on catalytic activity. J Phys Chem C 116(10):6222–6232. https://doi.org/10.1021/jp210555s
Kokalj A (2003) N2O interaction with Pd(110): cluster vs. slab model. Surf Sci 532–535:213–220. https://doi.org/10.1016/S0039-6028(03)00460-6
Hintz PA, Ervin KM (1995) Chemisorption and oxidation reactions of nickel group cluster anions with N2, O2, CO2, and N2O. J Chem Phys 103(18):7897–7906. https://doi.org/10.1063/1.470207
Parres-Esclapez S, Illán-Gómez MJ, Lecea SMD, Bueno-López A (2010) On the importance of the catalyst redox properties in the N2O decomposition over alumina and ceria supported Rh, Pd and Pt. Appl Catal B 96(3–4):370–378. https://doi.org/10.1016/j.apcatb.2010.02.034
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09. Revision B.01.01. Gaussian Inc., Wallingford
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys Rev B 46(11):6671–6687. https://doi.org/10.1103/physrevb.46.6671
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 78:1396. https://doi.org/10.1103/PhysRevLett.77.3865
Curtiss LA, McGrath MP, Blaudeau JP, Davis NE, Binning RC, Radom L (1995) Extension of Gaussian-2 theory to molecules containing third-row atoms Ga-Kr. J Chem Phys 103:6104–6113. https://doi.org/10.1063/1.470438
Cao XY, Dolg M (2002) Segmented contraction scheme for small-core lanthanide pseudopotential basis sets. J Mol Struct (Thoechem) 581:139–147. https://doi.org/10.1016/j.theochem.2003.12.015
Camacho-Mendoza RL, Cruz-Borbolla J (2020) Reaction mechanism for hydrogen production using the Pd4 cluster and formic acid by DFT. Chem Phys Lett 755:137794. https://doi.org/10.1016/j.cplett.2020.137794
Yu WL, Zuo HW, Lu CH, Li Y, Zhang YF, Chen WK (2015) Nitrous oxide decomposition catalyzed by Au19Pd and Au19Pt clusters. Acta Phys Chim Sin 31(3):425–434. https://doi.org/10.3866/PKU.WHXB201501191
Derdare M, Boudjahem AG, Boulbazine M (2022) Adsorption and decomposition mechanism of N2O molecule over MC23 (M=Ru, Mn, V, Pd, and Rh) nanoclusters: a comparative DFT investigation. Struct Chem 33:2043–2062. https://doi.org/10.1007/s11224-022-01984-2
Acknowledgements
This work is supported by the Science and Technology Research Program of Chongqing Municipal Education Commission (KJQN202101517).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, X., Zeng, W., Duan, H. et al. Density functional theory study of N2O decomposition catalyzed by Pd4−/0/+ clusters. Reac Kinet Mech Cat 136, 1933–1943 (2023). https://doi.org/10.1007/s11144-023-02456-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02456-2