Abstract
This work describes the isomerization of S-carvone using a natural zeolite—clinoptilolite as the catalyst. The isomerization of S-carvone was carried out at the catalyst content in the reaction mixture from 5 to 15 wt%, in a temperature range of 190–210 °C and for the reaction time from 60 to 300 min. The main product of the isomerization of S-carvone was aromatic alcohol with many practical applications—carvacrol. The use of the most favorable reaction conditions (the reaction time of 3 h, the temperature of 210 °C and the catalyst content 15 wt%) allowed to obtain this compound with high yield amounted to about 90 mol%. The S-carvone isomerization is an example of environmentally friendly process because it does not use any solvents, S-carvone can be separated from cheap cumin waste (renewable biomass) and a cheap zeolite of natural origin—clinoptilolite can be is used as the catalyst.
Graphic abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In modern research on the processes of isomerization and oxidation of olefinic compounds, there is a clear tendency to use organic raw materials of natural origin, especially waste plant sources (biomass). This trend also applies to catalysts, because a lot of research shows that porous materials of natural origin (minerals) can also be active catalysts for isomerization and oxidation processes. The use of reagents of natural origin (obtained from plant material) and minerals as catalysts often reduces the costs of running the process and perfectly fits the idea of sustainable development in the organic industry [1,2,3,4,5].
An example of catalyst of natural origin is a natural zeolite—clinoptilolite. Clinoptilolite is widely distributed zeolite belonging to the heulandite group. It can be described by the following formula: (Na,K,Ca)4Al6Si30O72·24H2O. A characteristic feature of clinoptilolite, distinguishing this mineral from other zeolites, is a high Si/Al ratio (> 4), as well as its high thermal stability. The clinoptilolite structure is two-dimensional and consists of three types of channels. The channels A are made of 10-membered rings with a diameter of 0.31–0.76 nm. Channels C are bounded by 8-membered rings with a diameter of 0.26–4.7 nm and are parallel to channels A. However, channels B like channels C are bounded by 8-membered rings and intersect the channels A and B [6, 7]. In the structures of zeolites there are present active centers (acid–base and oxidation-reducing). These active centers are responsible for the unique adsorptive and catalytic properties of zeolites [8].
A number of studies have been carried out which show the excellent catalytic properties of clinoptilolite, for example this zeolite was used in the α-pinene isomerization process. In this process, two main products were formed: limonene and camphene [9]. The isomerization of 1-butene to isobutene is another example of the use of clinoptilolite in catalytic processes. However, cobalt-modified clinoptilolite was used in this case [10]. A very interesting example of the use of clinoptilolite, taking into account environmental protection, is the catalytic degradation of polymers such as polyethylene, polypropylene and polystyrene (catalytic degradation allowed to obtain styrene with a selectivity of 54%) [11].
Cumin (Carum calvi L.) is a herb originating in Europe, West Asia and North Africa. The fruit of cumin is a cleavage consisting of two separate achenes. Cumin fruit and the oil that is obtained from them is often used as an additive to ice cream, sweets, pastries, meat, cheese, alcoholic and non-alcoholic beverages. The essential oil obtained from cumin seeds consists of two monoterpenes: R-limonene and S-carvone, while R-limonene is an intermediate in the biosynthesis of S-carvone (this assumption was confirmed by observations which show that during plant growth the percentage of S-carvone in the fruit increases in favor of the percentage of R-limonene) [12]. Essential oil containing S-carvone and R-limonene can also be obtained from cumin waste that remains after cleaning the fruit. Such waste includes all parts of the plant (including stems and fruits). Cumin fruits contain from 3 to 7% of essential oil. The use of cumin waste, which is a much cheaper raw material, allows to obtain oil in an amount of 1% of the weight of the waste. The most effective method of obtaining this oil is the method of the steam distillation.
The main product of S-carvone isomerization is carvacrol. Carvacrol is a monoterpene belonging to the phenols group. This compound often appears in essential oils next to thymol, which is its isomer (Fig. 1).
Both compounds have similar properties and have been used in folk medicine for centuries. Carvacrol has antibacterial, antifungal, analgesic, antioxidant and antiparasitic properties. This compound has a strong effect on S. Aureus kernels and E. Cola sticks. The performed studies showed that carvacrol and its derivatives are potent against Mycobacterium tuberculosis, which causes tuberculosis [13, 14]. Studies are known in which the anticancer properties of carvacrol have been demonstrated. This compound showed efficacy against mouse leukemia and was also cytotoxic against colon cancer cells. In addition, carvacrol was found to be cytotoxic to melanoma cells. Interestingly, this cytotoxicity decreased when carvacrol appeared in combination with vitamin C or vitamin E. In other studies, carvacrol anticancer properties were tested in the treatment of lung cancer [14]. Carvacrol derivatives also have antitumor activity (Fig. 2). Particularly noteworthy is derivative 2, which showed the best antioxidant properties, which makes it an effective antioxidant with low cytotoxic effect and with potential use in the therapy of disorders associated with oxidative stress [15, 16].
The first isomerization of S-carvone was carried out by Ritter and Ginsburg in 1950. They used 40% H2SO4 in the studies. The reaction was carried out in a glass flask, heating the reaction mixture under reflux for 3 h. After this time, carvacrol was obtained with a yield of 61%. In other studies, 6 M H2SO4 was used, after 35 min of isomerization performing under reflux, the product was obtained with the yield from 60 to 80% [17,18,19]. In 1995, the isomerization of S-carvone was carried out using microwaves, in which carvacrol was obtained with 85% yield after 35 min of the process performing. The isomerization reaction was carried out at 180 °C, at the pressure of 3300 hPa and in the presence of 1,4-dioxane. In this process p-toluenesulfonic acid was used as the catalyst [20]. Research has also been conducted into the isomerization of R-carvone over a heterogeneous catalyst—amberlyst 15 (sulfone resin). During test at 75 °C and for the catalyst content of 12.5 mol%, the carvacrol yield was 98% after the reaction time of 10 min. The reaction was carried out in a continuous reactor [21]. Isomerization of S-carvone was also carried out in autoclave using montmorillonite as the catalyst and in the presence of toluene as the solvent. After the reaction time of 5 h and for the temperature of 150 °C, carvacrol was obtained with the yield of 98.3% [22]. In another studies a solution of sodium iodide in acetonitrile was added to a mixture of S-carvone with chloro(triethyl)silane and triethylamine. After the reaction time of 4 h and at room temperature, carvacrol was obtained with yield of 67% [23].
Summing up the previous literature reports on the isomerization of S-carvone, it can be stated that until now organic solvents such as acetonitrile, 1,4-dioxane or toluene have been used in this reaction, as well as a highly corrosive sulfuric acid as the catalyst. In addition, an inert gas atmosphere, pressure apparatus (autoclaves) and microwave radiation (this method allows for obtaining high product yield in a very short time) were used. Among the heterogeneous catalysts tested in the S-carvone isomerization, very good results were obtained over amberlyst 15 (high yield of product at low temperature and for short reaction time). But it is known that the ion-exchange resin catalysts (amberlyst 15) are deactivated after a few cycles. The methods of the regeneration of such catalysts by removing physically adsorbed impurities are known, but these methods are not always effective. These impurities can be removed by the solvent extraction and acid–alkali treatments. Moreover, it is also possible the phenomenon of grafting of the organic compound to the residual double bonds in the structure of resin. The grafted organic compounds can be removed by treatment with ozone and chlorine dioxide solutions, but this method does not completely restore the initial catalyst activity [24]. On the other hand, it is known that zeolite catalysts can be easily regenerated by the calcination at raised temperature and by the solvent extraction. These methods allow to remove tars from pores of the catalyst, and are very effective. They allow to restore the activity of the zeolite catalysts and it can be used many times. In case of zeolite catalysts the problem can be leaching of metals from the structure of zeolite but it can be omitted by appropriate choosing the conditions of reaction performing [25, 26]. In addition, it should also be emphasized that clinoptilolite is a cheap and readily available porous material compared to other synthetic materials used in organic reactions as catalysts.
The purpose of this work was to examine the possibility of carrying out the isomerization of S-carvone to carvacrol over the natural zeolite – clinoptilolite as the catalyst. The studies were performed without the use of any organic solvents, in glass apparatus and at the atmospheric pressure. During the research, the influence of three parameters on the course of the isomerization of S-carvone was determined: temperature (190–210 °C), amount of catalyst (5–15 wt%) and reaction time (60–300 min). The results obtained during catalytic tests allowed to determine the most favorable values of these parameters taking into account mainly the yield of carvacrol and conversion of S-carvone.
Experimental section
Raw materials
For the isomerization of S-carvone, the following raw materials were used: S-( +)-carvone (> 96%, Acros Organics, USA) and natural clinoptilolite (zeolite) as the catalyst (95% of clinoptilolite, Turkey). In the gas chromatography analyses as the template of carvacrol the wild oregano oil was used (content of carvacrol 90%, Avitale, Poland).
A full characteristic clinoptilolite which was used in our studies was presented in our previous work [27]. In addition, we determined the total number of acidic sites (the sum of Brønsted and Lewis acidic sites) in the clinoptilolite by the method described by Vilcocq et al. [28].
The method of the isomerization of S-carvone over the clinoptilolite as the catalyst
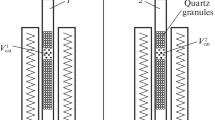
The influence of the reaction time, temperature, and clinoptilolite content on course of S-carvone isomerization was studies in a glass reactor with the capacity of 25 cm3. The glass reactor was equipped with a reflux condenser and a magnetic stirrer with a heating plate. Raw materials were introduced into the reactor in the following order: first 5.00 g of S-carvone and next appropriate amount of clinoptilolite (0.25 g (5 wt%), 0.5 g (10 wt%) or 0.75 g (15 wt%)). The reactor was placed in the paw and then immersed in an oil bath before stirring was started (500 rpm). S-carvone isomerization was tested at the following temperatures: 190 °C, 200 °C, and 210 °C. Samples of the post-reaction mixtures for gas chromatography analyses were taken for the following reaction time: 60 min, 120 min, 180 min, 240 min, and 300 min. To separate the catalyst from the post-reaction mixture, the reaction mixture was centrifuged.
The identification of products of S-carvone isomerization by the gas chromatography method
The qualitative and quantitative analyses of the post-reaction mixtures were made by the gas chromatography method (GC) with the FOCUS apparatus from Thermo Company (Waltham, MA USA). This apparatus was equipped with a flame-ionization detector (FID), a capillary column ZEBRON ZB-WAXplus type (0.32 mm × 30 m × 0.5 μm, filled with polyethylene glycol), and an autosampler. The analysis conditions were as follows: helium pressure 60 kPa, temperature of the injection port 240 °C, and detector temperature 250 °C. The thermostat temperature raised according to the following program: isothermally to 60° for 2 min, increase of temperature at a rate of 10 °C/min to 240 °C, isothermally to 240 °C for 4 min, and finally cooling to 60 °C. The samples for GC analysis were prepared in this way: first, the catalyst was separated from the post-reaction mixture with the help of a centrifuge; next, 0.250 g of the obtained solution was diluted with 0.750 g of acetone. The quantitative analyses were made with help of the external standard method. The conversion of S-carvone and yield of carvacrol was calculated based on chromatographic analyzes for each synthesis. The method of calculating these main values describing the process was as follows:
Results and discussion
In our earlier work, we included the full characteristics of clinoptilolite also used in the studies presented in this work [27]. It showed that the main elements occurring in clinoptilolite were: Si (32.7%), Al (about 5.5%), Ca (3.2%), K (2.1%) and Fe (2.0%)—the SEM–EDX method. The elemental map showed the spatial distribution of elements and all elements were incorporated uniformly in the structure of clinoptilolite. The SEM image showed that clinoptilolite was characterized by platy crystals of various sizes. The XRD pattern of clinoptilolite showed that this material was well crystallized and was well fitted to the literature data. The N2 adsorption/desorption isotherm prepared at 77 K for clinoptilolite showed that the isotherm for this porous material was of mixed types II and IV with an H3-type hysteresis loop [27]. In addition, we determined by the titration method (for this article only) the total number of acidic sites (the sum of Brønsted and Lewis acidic sites) in the clinoptilolite which we used. For these measurements we used the method described by Vilcocq et al. [28]. The titration of the acid sites performed for the studied sample of clinoptilolite allowed to obtain the following result: 0.22 mmolH+gcat−1. For comparison, we also carried out such a determination for synthetic zeolite, i.e. for the TS-1 catalyst, which we also use in the isomerization and oxidation processes we have studied. We obtained the following result for this catalyst: 0.89 mmolH+gcat−1. Comparison of these results shows that in the case of the synthetic zeolite catalyst (TS-1) it is possible to obtain greater amount of active acid sites. Fewer active sites, however, did not eliminate the high activity of clinoptilolite in the isomerization process which we investigated. On the contrary, this number of acid centers is sufficient to achieve high raw material conversions and high yields of the main product, as we shown below in this work.
The performed research allowed to obtain carvacrol as the main product during S-carvone isomerization over the clinoptilolite as the catalyst. This reaction is presented in Fig. 3.
In addition to the main product (carvacrol), small amounts of oxygenated and polymeric products were formed in this isomerization process.
Table 1 presents the changes of conversion of S-carvone during the studies on the isomerization of S-carvone.
It results from Table 1 that for the lowest catalyst content (5 wt%) and for the lowest process temperature (190 °C), the conversion of S-carvone increases with increasing the reaction time from 8.61 mol% (reaction time 60 min) to 31.14 mol% (reaction time 300 min). The highest S-carvone conversion values were observed for the highest temperature at which the process was carried out (210 °C) and for the highest catalyst content (15 wt%). In addition, at this temperature and at the highest catalyst content the highest S-carvone conversion was achieved in the shortest time. This conversion increased from 93.23 mol% (reaction time 60 min) to 100.00 mol% (reaction time 300 min). The comparison of the results obtained shows that both the catalyst content and the temperature of the process have a significant impact on the conversion of S-carvone. For the catalyst content of 10 wt% and at the temperature of 190 °C, the conversion of S-carvone is about 30 mol% lower in comparison to the conversion of this compound at the same catalyst content and at higher temperature (200 °C). At the lowest temperature (190 °C), the effect of catalyst content on S-carvone conversion is the most noticeable. The comparison the results obtained for this temperature shows that for the catalyst content of 5 wt%, the conversion of S-carvone is about 50 mol% lower in comparison to the conversion of this compound at the same temperature, but with the highest catalyst content (15 wt%). The obtained results show that increase in the content of the catalyst and simultaneously in the amount of active centers (Brønsted and Lewis) causes a greater availability of these active centers for the organic molecules which take part in the isomerization reaction. At the same time, the increase in temperature accelerates the diffusion of these molecules into the catalyst pores, which also facilitates the access of these molecules to the active centers of the catalyst.
Table 2 presents the changes of the yield of carvacrol and the total by-products yield during the studies on the conversion S-carvone.
Table 2 shows that all tested parameters have a significant impact on the yield of carvacrol, which is the main product of the studied process. It is worth noting that at higher isomerization temperatures (200 °C and 210 °C), the amount of by-products in the reaction mixture increases significantly. This is probably due to the intensification of polymerization and oxidation processes at these temperatures. At the lowest catalyst content (5 wt%) at 190 °C, the yield of by-products amounts to 3.63 mol%, while at 200 °C it increases almost 5 times to 19.36 mol% and at 210 °C almost 10 times to 34.52 mol% in comparison to the process carried out at the lowest tested temperature. Similar observation can be made for the catalyst content of 10 wt%, because at 190 °C the yield of by-products is 14.50 mol%, while at 200 °C it increases almost twofold to 27.96 mol%, similarly to the temperature of 210 °C, at which it amounts to 31.63 mol%. The exception is the synthesis which was carried out at the temperature of 210 °C and at the catalyst content of 15 wt%, in which the by-product yields of 10.99 mol% is achieved (at the temperature of 190 °C, the yield of by-products was 16.01 mol%, and at the temperature of 200 °C, it achieved 25.83 mol%).
The highest carvacrol yield was obtained at the temperature of 210 °C, for the highest catalyst content (15 wt%) and for the reaction time of 300 min–89.01 mol%. However, after 120 min of running the process at this temperature and with the catalyst content of 15 wt%, the carvacrol yield was 76.17 mol% and it was the highest yield of this product in comparison to previously conducted syntheses at lower temperatures or for lower catalyst contents, where the carvacrol yield did not exceed 70 mol%.
For better interpretation, the obtained results were also presented in the graphical form showing the dependence of: the conversion of S-carvone and the yield of carvacrol on the reaction time and the catalyst content at the temperature of 190 °C (Fig. 4) and 210 °C (Fig. 5), and the yield of carvacrol on the temperature and reaction time for the catalyst content 5 and 15 wt% (Figs. 6 and 7).
The influence of the reaction time and catalyst content on the conversion of S-carvone and the yield of carvacrol for the temperature of 190 °C. Experimental conditions: atmospheric pressure, 5 g of S-carvone, temperature 190 °C, amounts of clinoptilolite (catalyst) 0.25 g (5 wt% in relation to S-carvone), 0.5 g (10 wt% in relation to S-carvone) and 0.75 g (15 wt% in relation to S-carvone)
The influence of the reaction time and catalyst content on the conversion of S-carvone and the yield of carvacrol for the temperature of 210 °C. Experimental conditions: atmospheric pressure, 5 g of S-carvone, temperature 210 °C, amounts of clinoptilolite (catalyst) 0.25 g (5 wt% in relation to S-carvone), 0.5 g (10 wt% in relation to S-carvone) and 0.75 g (15 wt% in relation to S-carvone)
The influence of the reaction time and temperature on the yield of carvacrol. Amount of the catalyst was 0.75 g (15 wt% in relation to the mass of S-carvone). Experimental conditions: atmospheric pressure, 5 g of S-carvone, temperatures: 190 °C, 200 °C and 210 °C, amounts of clinoptilolite (catalyst) 0.75 g (15 wt% in relation to S-carvone)
Figs. 4 and 5 show that the yield of carvacrol (for the isomerization carried out at 190 °C and 210 °C and with the catalyst content of 5, 10 and 15 wt%) gradually increased with the prolongation of the reaction time from 60 to 300 min. At the temperature of 190 °C, the values of conversion of S-carvone were close to the values of the yield of carvacrol, which indicates high main product selectivity and low by-products yield. At higher temperatures, the yield of carvacrol was higher (210 °C), however, the differences between the values of the yield carvacrol and the conversion of S-carvone conversion were greater (Fig. 5). Figs. 4 and 5 also showed that the yield of carvacrol depended on the catalyst content, as the higher catalyst content in the reaction mixture provided for a greater number of active centers at which the isomerization of S-carvone can occur.
Figs. 6 and 7 show that differences in the yield of carvacrol values are particularly evident at the beginning of the process. The yield of carvacrol was 4.96 mol% after the reaction time of 60 min at 190 °C, while at the same time at 210 °C, the yield of carvacrol was nearly six times higher with the same catalyst content (5 wt%) (Fig. 6). Extending the reaction time reduced the difference between the product yield values in the tested temperature range, which was also be observed at the higher catalyst content (15 wt%)—Fig. 7. In addition, it can be seen that at the higher catalyst content (15 wt%), the product yields were very similar at 190 °C and 200 °C, however, increasing the process temperature by a further 10 °C allowed to obtain higher carvacrol yield. Most likely, this is due to the fact that the increase in the temperature increases the diffusion rate of S-carvone to the catalyst pores, thanks to which more organic compound molecules can bind to the active centers of the catalyst in a shorter time.
Taking into account the literature data [11, 12] and our previous studies [27, 29] on the isomerization of limonene we can proposed the following mechanism of the isomerization of S-carvone to carvacrol—Fig. 8.
In the first step a proton derived from a strongly acid site (Brønsted acid site) attaches to the double bound in the position of 8–9 of the carvone molecule and simultaneously a carbonium ion on the C8 atom is formed. Next, the removal of proton from C4 atom of the carvone molecule leads to the formation of double bond at the position of C4–C8. In the second step, proton derived from the Brønsted acid site attaches to the double bond in the position 4–8 and formation of carbonium ion on the C4 atom is observed. Next, proton from C5 atom is removed what leads to the formation of double bound at the position C4–C5. In the third step proton from the Brønsted acid site is attached to the carbonyl group, which causes hydroxyl group formation and simultaneously the carbonium ion on the C2 atom is formed. The elimination of proton from the C3 atom leads to the formation of double bound at the position C2–C3.
In the elimination of proton from the positions: 4, 5 or 3 can take part the Lewis acid site (the Lewis acid site of aluminum) [30,31,32]. An example of proton removal from position 5 is shown on Fig. 9 (similar can occur proton removal from positions 4 and 3).
Conclusions
The clinoptilolite catalyst enabled isomerization of S-carvone to carvacrol. These product has a lot of applications. An increase in the amount of catalyst used causes an increase in the yield of the carvacrol and shortens the reaction time needed for the obtaining of the main product. The highest yield of carvacrol was obtained at the highest temperature (210 °C) and for the highest catalyst content (15 wt%). It is worth noting that at these parameters which allow to achieve high yield of carvacrol (about 89 mol%) very small amounts of by-products were obtain (11 mol%). The yield of carvacrol higher than 80 mol% was achieved at these parameters after the reaction time 180 min. In comparison to the results obtained so far by other researchers, the yield of carvacrol in our studies is relatively high. Better results were obtained only by research groups that used in the isomerization process more complicated apparatus or organic solvents. However, research groups which used H2SO4 as the catalyst obtained worse results.
The presented S-carvone isomerization process is environment friendly for several reasons: (1) no organic solvents were used during isomerization, (2) the presented S-carvone isomerization method makes it possible to effectively use cumin waste—renewable biomass (S-carvone is the main component ( in addition to limonene) the oil obtained by the hydrodistillation of these wastes and after separation from limonene, it can be directly subjected to isomerization) and (3) a cheap zeolite catalyst of natural origin, such as clinoptilolite, is used for isomerization. In addition, the isomerization process described in this work does not require complicated equipment, and the reaction can be carried out without the use of an inert atmosphere. The low essential oil concentration in cumin fruits waste is not a disadvantage to implement this process at the industrial level. First of all, obtaining oil from cumin waste allows to manage this waste—a very large amount of this waste is generated because Poland is the largest producer of cumin in Europe. On the other hand, the residue after distilling off the oil (cumin pulp) can be used as an animal feed additive, or by means of pyrolysis it can be used to obtain carbon catalysts for oxidation and isomerization processes.
References
Higman CS, de Araujo MP, Fogg DE (2016) Tandem catalysis versus one-pot catalysis: ensuring process orthogonality in the transformation of essential-oil phenylpropenoids into high-value products via olefin isomerization–metathesis. Catal Sci Technol 6(7):2077–2084. https://doi.org/10.1039/C5CY02038G
Cui H, Zhang J, Luo Z, Zhao C (2016) Mechanisms into dehydroaromatization of bio-derived limonene to p-cymene over Pd/HZSM-5 in the presence and absence of H2. RSC Adv 6(71):66695–66704. https://doi.org/10.1039/C6RA17159A
Hassam M, Taher A, Arnott GE, Green IR, van Otterlo WAL (2015) Isomerization of allylbenzenes. Chem Rev 115(11):5462–5569. https://doi.org/10.1021/acs.chemrev.5b00052
Ciriminna R, Parrino F, De Pasquale C, Palmisano L, Pagliaro M (2018) Photocatalytic partial oxidation of limonene to 1,2 limonene oxide. Chem Commun 54:1008–1011. https://doi.org/10.1039/C7CC09788C
Fajdek-Bieda A, Wróblewska A, Miądlicki P, Szymańska A, Dzięcioł M, Booth AM, Michalkiewicz B (2019) Influence of technological parameters on the isomerization of geraniol using sepiolite. Catal Lett 150:1–11. https://doi.org/10.1007/s10562-019-02987-1
Sprynsky M, Buszewski B, Terzyk AP, Namiesnik J (2006) Study of the selection mechanism of heavy metal (Pb2+, Cu2+, Ni2+, and Cd2+) adsorption on clinoptilolite. J Colloid Interface Sci 304(1):21–28. https://doi.org/10.1016/j.jcis.2006.07.068
Ambrozova P, Kynicky J, Urubek T, Nguyen VD (2017) Synthesis and modification of clinoptilolite. Molecules 22(7):1107–1120. https://doi.org/10.3390/molecules22071107
Kasperkowski M (2014) Materiały mikro- i mezoporowate, jako napełniacze aktywne, PhD thesis, Poznan University of Technology, PL.
Allahverdiev AI, Irandoust S, Murzin YD (1999) Isomerization of α-pinene over clinoptilolite. J Catal 185(2):352–362. https://doi.org/10.1006/jcat.1999.2474
Lee HC, Woo HC, Chung SH, Kim HJ, Lee KH, Lee JS (2002) Effects of metal cation on the skeletal isomerization of 1-butene over clinoptilolite. J Ctatal 211(1):216–225. https://doi.org/10.1006/jcat.2002.3732
Lee SY, Yoon JH, Kim JR, Park DW (2002) Degradation of polystyrene using clinoptilolite catalysts. J Anal Appl Pyroly 64(1):71–83. https://doi.org/10.1016/S0165-2370(01)00171-1
Bouwmeester HJ, Gershenzon J, Konings MCJM, Croteau R (1998) Biosynthesis of the monoterpenes limonene and carvone in the fruit of caraway. Plant Physiol 117(9):901–912. https://doi.org/10.1104/pp.117.3.901
Hołderna-Kędzia E (2010) Działanie przeciwdrobnoustrojowe roślinnych pochodnych fenolu. Postępy Fitoterapii 1(3):3–8
Alokam R, Jeankumar VU, Sridevi JP, Matikonda SS, Peddi S, Alvala M, Yogeeswari P, Sriram D (2014) Identification and structure-activity relationship study of carvacrol derivatives as Mycobacterium tuberculosis chorismate mutase inhibitors. J Enzyme Inhib Med Chem 29(4):547–554. https://doi.org/10.3109/14756366.2013.823958
Rajput JD, Bagul SD, Hosamani AA, Patil MM, Bendre RS (2017) Synthesis, characterizations, biological activities and docking studies of novel dihydroxy derivatives of natural phenolic monoterpenoids containing azomethine linkage. Res Chem Intermed 43:5377–5393. https://doi.org/10.1007/s11164-017-2933-4
Mastelic J, Jerkovic I, Blazevic I, Poljak-Blazi M, Borovic S, Ivanc-Bace I, Smercki V, Zarkovic N, Brcić-Kostic K, Vikic-Topic D, Muller N (2008) Comparative study on the antioxidant and biological activities of carvacrol, thymol, and eugenol derivatives. J Agric Food Chem 56(11):3989–3996. https://doi.org/10.1021/jf073272v
Ritter J, Ginsburg D (1950) The action of f-butyl hypochlorite on α-pinene 1,2. J Am Chem Soc 72(6):2381–2384. https://doi.org/10.1021/ja01162a009
Singha B, Patiala J, Sharmaa P, Chandraa S, Kaula P, Maity S (2011) Role of acidity for the production of carvacrol from carvone over sulfated zircona. Indian J Chem Technol 18(1):21–28
Kjonaas WA, Mattingly SP (2005) Acid-catalyzed isomerization of carvone to carvacrol. J Chem Educ 82(12):1813–1814. https://doi.org/10.1021/ed082p1813
Raner KD, Strauss CR, Trainor RW, Thom JS (1995) A new microwave reactor for batchwise organic synthesis. J Org Chem 60(8):2456–2460. https://doi.org/10.1021/jo00113a028
Gozzi C, Convard A, Husset M (2009) Heterogeneous acid-catalysed isomerization of carvone to carvacrol. React Kinet Catal Lett 97:301–306
Liu X, Zhou Y, Zhang F, Wang K, Zhu Y, Wan M (2016) A synthetic method for carvacrol https://patents.google.com/patent/CN105523897A/en. Accessed 27 April 2016
Valeev RF, Bikzhanov RF, Selezneva NK, Gimalova FA, Miftakhov MS (2011) Synthesis of 6-hydroxycarvone derivatives and their oxidative decyclization with lead tetraacetate. Russ J Org Chem 47:1287–1292. https://doi.org/10.1134/S1070428011090041
Malshe VC, Sujatha ES (1997) Regeneration and reuse of cation-exchange resin catalyst used in alkylation of phenol. React Funct Polym 35(3):159–168. https://doi.org/10.1016/S1381-5148(97)00092-8
Wróblewska A, Makuch E (2013) Studies on the deactivation of Ti-MCM-41 catalyst in the process of allyl alcohol epoxidation. Pol J Chem Technol 4(15):111–115. https://doi.org/10.2478/pjct-2013-0078
Wróblewska A, Makuch E (2014) Regeneration of the Ti-SBA-15 catalyst used in the process of allyl alcohol epoxidation with hydrogen peroxide. J Adv Oxid Technol 17(1):44–52. https://doi.org/10.1515/jaots-2014-0106
Retajczyk M, Wróblewska A, Szymańska A, Michalkiewicz B (2019) Isomerization of limonene over natural zeolite-clinoptilolite. Clay Miner 54:121–129. https://doi.org/10.1180/clm.2019.18
Vilcocq L, Spinola V, Moniz P, Duarte LC, Carvalheiro F, Fernandes C, Castilho P (2015) Acid-modified clays as green catalysts for the hydrolysis of hemicellulosic oligosaccharides. Catal Sci Technol 5:4072–4080. https://doi.org/10.1039/c5cy00195
Retajczyk M, Wróblewska A (2019) Isomerization and dehydroaromatization of R(+)-limonene over the Ti-MCM-41 catalyst: effect of temperature, reaction time and catalyst content on product yield. Catalysts 9:1–11. https://doi.org/10.3390/catal9060508
Stanislaus A, Yeddanalpalli LM (1972) Vapor phase catalytic transformations of terpene hydrocarbons in the C10H16 series I. Isomerization of α-pinene over alumina. Can J. Chem. 50:61–74. https://doi.org/10.1139/v72-010
Detrekoy EJ, Jacobs PA, Kallo D, Uytterhoeven JB (1973) The nature of catalytic activity of hydroxyl groups in clinoptilolite. J Catal 32:442–452. https://doi.org/10.1016/0021-9517(74)90095-5
Grau RJ, Zgolicz PD, Gutierrez C, Taher HA (1999) Liquid phase hydrogenation, isomerization and dehydrogenation of limonene and derivatives with supported palladium catalysts. J Mol Catal A 148:203–214. https://doi.org/10.1016/S1381-1169(99)00108-9
Author information
Authors and Affiliations
Contributions
MR made isomerization’s in the laboratory. She prepared the GC analyses, calculated the main functions describing the studied process and took part in the interpretation of data for the work. She prepared the preliminary Polish version of this manuscript. AW took part in the interpretation of data for work, in the selecting appropriate parameters for next stages of studies, and she proposed the mechanism of the studied reaction. She corrected critically the preliminary version of this manuscript, including introduction, discussion of the results and conclusions. She has prepared the English version of this manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wróblewska, A., Retajczyk, M. The isomerization of S-carvone over the natural clinoptilolite as the catalyst: the influence of reaction time, temperature and catalyst content. Reac Kinet Mech Cat 130, 273–288 (2020). https://doi.org/10.1007/s11144-020-01781-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01781-0