Abstract
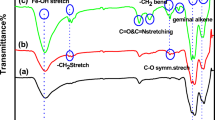
In the paper, the synthesis of the magnetic biocatalyst based on immobilized peroxidase is described. This catalyst was tested in the reaction of 2,3,6,-trimethylphenol oxidation with the help of hydrogen peroxide to 2,3,5-trimethylhydrochinone (vitamin E intermediate). In the work, the method for magnetic nanoparticle synthesis was chosen. To characterize the synthesized biocatalyst physical–chemical analysis was carried out: transmission electron microscopy, FTIR spectroscopy, the study of magnetic characteristics with vibration magnetometer, X-ray photoelectron spectroscopy, low-temperature nitrogen adsorption. The optimum conditions for the process of 2,3,6,-trimethylphenol oxidation in the presence of magnetic biocatalyst (the substrate initial concentration, temperature, pH).






Similar content being viewed by others
References
Soh N, Kaneko S, Uozumi K, Ueda T, Kamada K (2014) J Mater Sci 49:8010–8015
Bilal M, Rasheed T, Zhao Y, Iqbal HMN, Cui J (2018) “Smart” chemistry and its application in peroxidase immobilization using different support materials. Int J Bio Macromol. https://doi.org/10.1016/j.ijbiomac.2018.07.134
Bi S, Cui Y, Dong Y, Zhang N (2014) Biosens Bioelectron 53:207–213
Garcia J, Zhang Y, Taylor H, Cespedes O, Webb ME (2011) Nanoscale 3(9):3721–3730
Yang H, Gong C, Miao L, Xu F (2017) Int J Electrochem Sci 12:4958–4969
Golami-Borujeni F, Faramarzi MA, Nejatzadeh-Barandozi F, Mahvi AH (2013) Fresenius Environ Bull 22:739–744
Grosu EF, Carja G, Froidevaux R (2018) Chem Intermed 44:773
Vineh MB, Saboury AA, PoostchiA A, Rashid AM, Parivar K (2017) Stability and activity improvement of horseradish peroxidase by covalent immobilization on functionalized reduced graphene oxide and biodegradation of high phenol concentration. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2017.08.133
Ai J, Zhang W, Liao G, Xia H, Wang D (2017) NH2-Fe3O4@SiO2 supported peroxidase catalyzed H2O2 for degradation of endocrine disrupter from aqueous solution: roles of active radicals and NOMs. Chemosphere. https://doi.org/10.1016/j.chemosphere.2017.08.039
Bilal M, Iqba H, Rasheed T, Hu H (2017) Int J Biol Macromol 105:328–335
Li ZL, Cheng L, Zhang LW, Liu W, Ma WQ (2017) Preparation of a Novel Multi-walled-carbon-nanotube/cordierite composite support and its immobilization effect on horseradish peroxidase. Process Saf Environ Prot. https://doi.org/10.1016/j.psep.2017.02.021
Zheng Y, Zhaob S, Liu Y-M (2011) Analyst 136:2890–2892
Zhou Y, Zhou T, Zhou R, Hu Y (2014) Luminescence 29:338–343
Barbosa EF, Molina FJ, Lopes FM, Garcıa-Ruız PA, Caramori SS et al (2012) Immobilization of peroxidase onto magnetite modified polyaniline. ScientificWorld J. https://doi.org/10.1100/2012/716374
Song J, Shen H, Yang Y, Zhou Z, Su P et al (2018) Multifunctional magnetic particles for effective suppression of non-specific adsorption and coimmobilization of multiple enzymes by DNA directed immobilization. Mater Chem B. https://doi.org/10.1039/C8TB01842A
Samoilova N, Tikhonov V, Krayukhina M, Yamskov I (2014) J Appl Polym Sci 131:39663–39663
Bayramoglu G, Arica MY (2008) J Hazard Mater 156:148–155
Corgie SC, Kahawong P, Duan X (2012) Adv Funct Mater 22:1940–1951
Deepthi SS, Prasad E, Reddy BV (2014) Green Sustain Chem 4:15–19
Li Y, Liu W, Wu M, Yi Z, Zhang J (2007) J Mol Catal A 261:73–78
Palacio M, Villabrille PI, Romanelli GP (2012) Appl Catal A 417–418:273–280
Türk H (2008) Appl Catal A 340:52–58
Saux C, Pizzio LR, Pierella LB (2013) Appl Catal A 452:17–23
Koreniuk A (2016) Microporous Mesoporous Mater 229:98–105
Wang G, Wanga Y, Yaoa J, Li H (2019) Mol Catal 472:10–16
Laurent S, Forge D, Port M (2008) Chem Rev 108:2064–2110
Jadhav SA, Bongiovanni R (2012) Adv Mat Lett 3:356–361
Cheng C, Xuw F, Gu H (2011) NewJ Chem 35:1072–1079
Baranov DA, Gubin SP (2009) Nanosystems 1:129–147
Ma M, Zhang Y, Yu W, Shen H, Zhang H et al (2003) Coll Surf A 212:219–226
Silverstein RM, Webster FX, Kiemle DJ, Bryce DL (2011) Spectrometric identification of organic compounds. Wiley, Hoboken
NIST X-ray Photoelectron Spectroscopy Database Version 3.5 (2003) National Institute of Standards and Technology, Gaithersburg. https://srdata.nist.gov/xps/
Zhang S, Wu W, Xiao X, Zhou J, Ren F, Jiang C (2011) Nanoscale Res Lett 6:89
Malomo SO, Adeoye RI, Babatunde L, Saheed IA, Iniaghe MO et al (2011) Biokemistri 23:124–128
Schmid R, Sapunov VN (1982) Non formal kinetics. In search for chemical reaction pathways. Stanford University, Stanford
Yu F, Huang Y, Cole AJ, Yang C (2009) Biomaterials 30:4716–4722
Kholdeeva OA, Ivanchikova ID, Guidotti M, Ravasio N (2007) Green Chem 9:731–733
Rogozhin VV (2004) Peroxidase as a component of the antioxidant system of living organisms. GIORD, St. Petersburg
Henriksen A, Smith AT, Gajhede M (1999) J Biol Chem 274:35005–35011
Dawson J (1988) Science 240:433–439
Acknowledgements
The financial support was provided by the Russian Science Foundation (Grant No. 19-79-00134).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Grebennikova, O., Sulman, A., Matveeva, V. et al. Physical–chemical analysis and kinetics of the magnetic biocatalyst for 2,3,6,-trimethylphenol oxidation. Reac Kinet Mech Cat 130, 317–329 (2020). https://doi.org/10.1007/s11144-020-01762-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01762-3