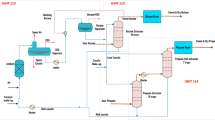
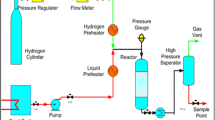
Abstract
A basic conversion model for hydrodesulfurization (HDS) is developed according to corresponding reaction process. Further improvement is conducted on the model considering the HDS characteristics and industrial demand. The model can quantitatively describe the effect of operational conditions, deactivation behavior and residual properties on HDS. By comparison with the experimental data, the calculated conversions are all found to have a total average relative deviation of less than 5%, presenting a good fit in relation to the experimental data. Moreover, the model can also accurately predict the performance of hydrodecarbonresidue and hydrodemetallization. Results indicate that the model has a high universality and practicability.




Similar content being viewed by others
Abbreviations
- \(C_S\) :
-
Contention of representative S compound (wt%)
- \(C_{{S_{0} }}\) :
-
Initial contention of representative S compound (wt%)
- \(Cz\) :
-
The contents of Catalyst surface active sites (mol/m2)
- \(C_{M}\) :
-
Adsorbent concentration (mol/m2)
- \(C_{{M_{0} }}\) :
-
Initial contention of representative metal compound (mg/kg)
- \(C_{{C_{0} }}\) :
-
Initial contention of representative carbon residue compound (wt%)
- \(C_{H}\) :
-
The contents of hydrogen atom (mol/m2)
- \(C_{L}\) :
-
Bituminous concentration (wt%)
- \(R\) :
-
Gas constant (8.314 J mol−1 K−1)
- \(E_{1}\) :
-
Activation energy of first step reaction (kJ/mol)
- \(E_{2}\) :
-
Activation energy of second step reaction (kJ/mol)
- \(C_{{H_{2} }}\) :
-
Hydrogen gas concentration (mol/m3)
- \(C_{{H_{20} }}\) :
-
Initial hydrogen gas concentration (mol/m3)
- \(\alpha_{1}\) and \(\alpha_{2}\) :
-
Average weights
- \(\tau\) :
-
Reaction time (h)
- \(T\) :
-
Reaction temperature (°C)
- \(E_{a}\) :
-
Relative apparent activation energy
- \(E_{a}^{'}\) :
-
Apparent activation energy (kJ/mol)
- \(\pi_{c}\) :
-
Active retention factor
- \(k_{d}\) :
-
Deactivation rate constant of covering
- \(t\) :
-
Running time (h or day)
- \(n_{0}\) :
-
The parameter of deactivation curve shape
- \(\omega\) :
-
The parameter of initial hydrogen concentration
- \(m_{0}\) :
-
The index of reaction time
- \(m_{1}\) :
-
The index of reaction time
- \(k_{0}^{{}}\) :
-
Pre-exponential factors (related)
- \(k_{0}^{'}\) :
-
The pre-exponential factor when t = 0
- \(\eta\) :
-
Internal diffusion efficient factor
- \(k_{{}}^{'}\) :
-
Rate coefficient when \(\eta\) = 1
- \(k\) :
-
Rate coefficient
- \(D_{e}^{{}}\) :
-
Effective diffusion coefficient
- \(\eta_{0}\) :
-
Coefficient
- \(N\) :
-
The parameter of internal diffusion resistance
- \(r_{0}\) :
-
The mesoporous radius of fresh catalyst (nm)
- \(k_{d}^{'}\) :
-
The channel reduction deactivation factor of catalyst
- \(n_{0}^{'}\) :
-
The shape parameter of deactivation curve
- \(V_{cat}\) :
-
Catalyst bed volume (m3)
- \(\lambda\) :
-
Hydrogen oil volume ratio (V/V)
- \(F_{oil}\) :
-
Flow of liquid oil into the reactor (ton/h)
- \(C_{20}\) :
-
Initial concentration of H2
- \(P\) :
-
Operation pressure (MPa)
- \(K_{H}\) :
-
Dissociation adsorption constant of H2
References
Barkat M, Nibou D, Chegrouche S, Mellah A (2009) Chem Eng Process 48(1):38–47
Boehme TR, Onder CH, Guzzella L (2008) Comput Chem Eng 32(10):2445–2454
Jaree A, Boonsomlanjit B, Limtrakul J (2008) Comput Chem Eng 32(12):2897–2902
Alvarezmajmutov A, Gieleciak R, Chen J (2015) Energy Fuels 29(12):7931–7940
Al-Barood A, Qabazard H, Stanislaus A (2005) Liq Fuels Technol 23(7–8):12
Ferreira C, Tayakout-Fayolle M, Guibard I, Lemos F (2014) Fuel 129(7):267–277
Vonortas A, Papayannakos N (2014) Ind Eng Chem Res 53(23):9646–9652
Doukeh R, Bombos M, Trifoi A, Mihai O, Popovici D (2018) C R Chim 21(3–4):277–287
Tang X, Li S, Yue C, He J, Hou J (2013) Oil Shale 30(4):517–535
Yang Y, Dai F, Li C, Xiang S, Yaseen M (2017) Energy Fuels 31(5):5491–5497
Rodríguez MA, Elizalde I, Ancheyta J (2012) Fuel 100(4):152–162
Lente G (2015) J Math Chem 53(4):1172–1183
Lente G (2012) J Chem Phys 137(16):164101
Albazzaz H, Marafi AMJ, Ma X, Ansari T (2017) Energy Fuels 31(1):831–838
Mederos FS, Ancheyta J, Elizalde I (2012) Appl Catal A Gen 425:13–27
Stanislaus A, Marafi A, Rana MS (2010) Catal Today 153(1):1–68
Kam EKT, Al-Shamali M, Juraidan M (2005) Energy Fuels 19(3):753–764
Marafi A, Kam E, Stanislaus A (2008) Fuel 87(10–11):2131–2140
Alonso F, Ancheyta J (2017) Catal Today 305:203–211
Nguyen TTH, Teratani S, Tanaka R, Endo A, Hirao M (2017) Energy Fuels 31(5):5673–5681
Morales-Valencia EM, Castillo-Araiza CO, Giraldo SA, Baldovinomedrano VG (2018) ACS Catal 8:3926–3942
Niu M, Zheng H, Sun X, Zhang S, Li D (2017) Energy Fuel 31(5):5441–5447
Li Q, Zhang Y, Chen S, Fang W, Yang Y (2011) Chin J Catal 32(3):446–450
Elizalde I, Ancheyta J (2014) Catal Today 220:221–227
Wood J, Gladden LF (2003) Appl Catal A Gen 249(2):241–253
Ancheyta J, Betancourt G, Centeno G, Marroquín G, Alonso F, Garciafigueroa E (2002) Energy Fuels 16(6):1438–1443
Wu H, Duan A, Zhao Z, Xu C, Jiang G, Liu J, Wei Y, Li J, Chi K, Guo J (2017) RSC Adv 7(70):44340–44347
Li C, Chen YW, Yang SJ, Wu JC (1993) Ind Eng Chem Res 32(8):1573–1578
Kathawalla IA, Anderson JL (1988) Ind Eng Chem Res 27(5):866–871
Acknowledgements
This work is supported by the National Natural Science Foundation of China (21703179, 21773194 and 21473143) and the Fundamental Research Funds for the Central Universities of China (20720170103).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, X., Yang, Z., Yuan, S. et al. Development of a modified kinetic model for residual oil hydroprocessing. Reac Kinet Mech Cat 126, 921–937 (2019). https://doi.org/10.1007/s11144-019-01556-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-019-01556-2