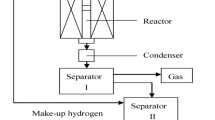
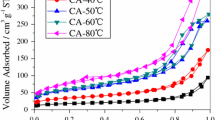
Abstract
The H6P2W18O62@MCM-41 catalyst (C-1) modified by dodecyltrimethoxysilane (DTMS) showed excellent catalytic performance during the dehydration of castor oil to produce dehydrated castor oil (DCO). The castor oil conversion and the DCO selectivity on H6P2W18O62-DTMS@Zr-MCM-41 (C-2) are higher than those of C-1. The dispersity of H6P2W18O62 is remarkably improved by an appropriate amount of DTMS modification on the C-2 because of the steric effect between the DTMS and H6P2W18O62. Therefore, the total acidic amount of C-2 is more than that of C-1, but the acidic intensity is weaker than that of C-1. The more total amount of acidity is good for improving the castor oil conversion, and the weaker acid strength leads to less carbon deposition. The amount of Brønsted acid sites is higher than that of C-1. However, the amount of Lewis acid sites is less than that of C-1, so the ration of B/(B + L) is higher than that of C-1, which is good for improving the DCO selectivity. The C-2 catalyst has proper hydrophobicity by grafting the silane group of DTMS on the surface of MCM-41, which can suppress the leaching of H6P2W18O62, because the water produced in reaction is not easily adsorbed on the surface of the catalyst. Proper lipophilicity is conducive to the absorption of castor oil to the surface of the catalyst, which can accelerate the reaction. The stability of C-2 is obviously higher than that of C-1, because the coke deposited and W elemental leaching on C-1 are more serious than those of C-2. Under the optimized reaction conditions: 220 °C, 2.8 wt% amount of C-2, H6P2W18O62 loading of 12 wt%, DTMS loading of 3.5 wt%, the iodine and hydroxyl value of product are 148.6 and 8.9, respectively.












Similar content being viewed by others
References
Ogunniyi DS (2006) Bioresource Technol 97(9):1086–1091
Naughton FC (1974) J Am Oil Chem Soc 51:65–71
Meller E, Green U, Aizenshtat Z, Sasson Y (2014) Fuel 133:89–95
Azcan N, Demirel E, Yılmaz O, Erciyes AT (2011) Ind Eng Chem Res 50:398–403
Achaya KT (1971) J Am Oil Chem Soc 48:758–763
Guner FS (1997) J Am Oil Chem Soc 74:409–412
Bolley DS (1959) J Am Oil Chem Soc 36:518–523
Yan CX, Ding JF, Ma TL, Shao R, Xu W, He J, Wang PF (2017) Zeitschrift für anorganische und allgemeine Chemie 643:772–779
Chen XY, Jia M, Liu GZ, Zhang XW, Wang L, Mi ZT (2010) Appl Surf Sci 256:5856–5861
Zou JJ, Xu Y, Zhang XW, Wang L (2012) Appl Catal A 421:79–85
Ma TL, Yun Z, Xu W, Chen LG, Li L, Ding JF, Shao R (2016) Chem Eng J 294:343–352
Ma TL, Ding JF, Shao R, Xu W, Yun Z (2017) Chem Eng J 316:797–806
ISO 3961 (1996) Standardization administration of the People’s Republic of China
Hartman L, Lago RC, Azeredo LC, Azeredo MA (1987) Analyst 112:145–147
Karthikeyan G, Pandurangan A (2009) J Mol Catal A 311:36–45
Matkovic SR, Valle GM, Gambaro LA, Briand LE (2008) Catal Today 133:192–199
Luo SS, Fan GZ, Luo M, Li JF, Song GS (2016) J. CO2 Util 14:23–30
Yasmina T, Müllera K (2010) J Chromatogr A 1217:3362–3374
Hu Y, He YY, Wang XW, Wei CH (2014) Appl Surf Sci 311:825–830
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS (1992) Nature 359:710–712
Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CTW, Olson DH, Sheppard EW, McCullen SB, Higgins JB, Schlenker JL (1992) J Am Chem Soc 114(27):10834–10843
Berteau P, Delmon B (1989) Catal Today 5:121–137
Khder AERS, Hassan HMA, El-Shall MS (2012) Appl. Catal. A 411:77–86
Acknowledgements
We are grateful for the financial support of the National Natural Science Foundation of China (No. 21303154), National high Technology Research and Development Program (2015AA021003) and the joint research fund between Collaborative Innovation Center for Ecological Building Materials and Environmental Protection Equipments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ding, J., Ma, T., Yan, C. et al. Dehydration of castor oil over H6P2W18O62@MCM-41. Reac Kinet Mech Cat 125, 1007–1021 (2018). https://doi.org/10.1007/s11144-018-1450-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-018-1450-9