Summary
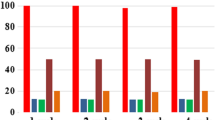
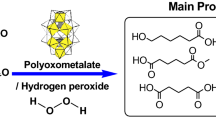
Epoxidation of natural terpene (+)-carvone by the system consisting of a catalyst, oxalic acid (co-catalyst) and H2O2 (70% aqueous solution; oxidant) was studied and factorial design methods were applied for the optimization of this reaction. A dinuclear manganese(IV) complex [LMn(O)3MnL](PF6)2 (L = 1,4,7-trimethyl-1,4,7-triazacyclononane) was used as a catalyst, and acetonitrile was employed as a solvent. An analysis by methods of the complete 24 factorial design showed that an increase in the catalyst concentration gives a strong positive effect on the carvone conversion and selectivity. Hydrogen peroxide has a smaller positive effect on the conversion, but at high concentration, H2O2 leads to some decrease in the selectivity. An increase in the oxalic acid concentration has a beneficial effect on the conversion, but does not affect the selectivity.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mandelli, D., Steffen, R. & Shul'pin, G. Carvone epoxidation by system “hydrogen peroxide-[Mn 2 L 2 O 3 ][PF 6 ] 2 (L = 1,4,7-trimethyl-1,4,7-triazacyclononane)-carboxylic acid”: a combinatorial approach to the process optimization . React Kinet Catal Lett 88, 165–173 (2006). https://doi.org/10.1007/s11144-006-0124-1
Issue Date:
DOI: https://doi.org/10.1007/s11144-006-0124-1