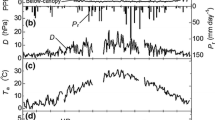
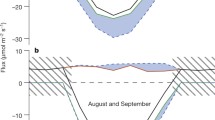
Abstract
Nitrogen allocated to the photosynthetic apparatus and its partitioning into different photosynthetic components is crucial for understanding plant carbon gain and plant productivity. It is known that photosynthetic nitrogen content and partitioning are controlled by both environmental and vegetation factors and have versatile and dynamic responses. However, such responses are greatly simplified in most current gas exchange models, in which only a prescribed relationship is commonly applied to describe the effect of nitrogen on photosynthesis and with limited model performance. While within-canopy variation at a specific time in leaf photosynthetic nitrogen content and partitioning has been studied previously, far less attention has been paid to the seasonal dynamics of photosynthetic nitrogen content and partitioning, which is especially critical to deciduous forests. In this study, we integrated long-term field observations in deciduous forests in Japan to determine seasonal patterns of photosynthetic nitrogen content and partitioning (rubisco, electron transport, and light capture) and to examine how photosynthetic nitrogen content and partitioning varied seasonally in deciduous forest canopies growing at different altitudes. The results demonstrated that there were remarkable seasonal variations in both photosynthetic nitrogen content and partitioning in deciduous forests along the altitudinal gradient. Moreover, photosynthetic nitrogen use efficiency was well explained by nitrogen partitioning rather than total leaf nitrogen. These results suggest that seasonal patterns of nitrogen partitioning should be integrated into ecosystem models to accurately project emergent properties of ecosystem productivity on local, regional, and global scales.







Similar content being viewed by others
References
Ali AA, Xu C, Rogers A et al (2016) A global scale mechanistic model of photosynthetic capacity (LUNA V1.0). Geosci Model Dev 9:587–606
Bertheloot J, Andrieu B, Martre P (2012) Light-nitrogen relationships within reproductive wheat canopy are modulated by plant modular organization. Eur J Agron 42:11–21
Bonan GB, Lawrence PJ, Oleson KW et al (2011) Improving canopy processes in the community land model version 4 (CLM4) using global flux fields empirically inferred from FLUXNET data. J Geophys Res 116:G02014
Buckley TN, Cescatti A, Farquhar GD (2013) What does optimization theory actually predict about crown profiles of photosynthetic capacity when models incorporate greater realism? Plant Cell Environ 36:1547–1563
Cai ZQ, Chen YJ, Bongers F (2007) Seasonal changes in photosynthesis and growth of Zizyphus attopensis seedlings in three contrasting microhabitats in a tropical seasonal rain forest. Tree Physiol 27:827–836
Clark DB, Mercado LM, Sitch S et al (2011) The Joint UK land environment simulator (JULES), model description—Part 2: carbon fluxes and vegetation dynamics. Geosci Model Dev 4:701–722
Coste S, Roggy JC, Imbert P et al (2005) Leaf photosynthetic traits of 14 tropical rain forest species in relation to leaf nitrogen concentration and shade tolerance. Tree Physiol 25:1127–1137
Delagrange S (2011) Light- and seasonal-induced plasticity in leaf morphology, N partitioning and photosynthetic capacity of two temperate deciduous species. Environ Exp Bot 70:1–10
Dietze MC (2014) Gaps in knowledge and data driving uncertainty in models of photosynthesis. Photosynth Res 119:3–14
Evans JR, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24:755–767
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Feng YL, Fu GL (2008) Nitrogen allocation, partitioning and use efficiency in three invasive plant species in comparison with their native congeners. Biol Invasions 10:891–902
Feng YL, Auge H, Ebeling SK (2007) Invasive Buddleja davidii allocates more nitrogen to its photosynthetic machinery than five native woody species. Oecologia 153:501–510
Feng YL, Lei YB, Wang RF et al (2009) Evolutionary tradeoffs for nitrogen allocation to photosynthesis versus cell walls in an invasive plant. Proc Natl Acad Sci USA 106:1853–1856
Foley S, Rivard B, Sanchez-Azofeifa GA, Calvo J (2006) Foliar spectral properties following leaf clipping and implications for handling techniques. Remote Sens Environ 103:265–275
Ghimire B, Riley WJ, Koven CD et al (2017) A global trait-based approach to estimate leaf nitrogen functional allocation from observations. Ecol Appl 27:1421–1434
Grassi G, Vicinelli E, Ponti F et al (2005) Seasonal and interannual variability of photosynthetic capacity in relation to leaf nitrogen in a deciduous forest plantation in northern Italy. Tree Physiol 25:349–360
Guan LL, Wen DZ (2011) More nitrogen partition in structural proteins and decreased photosynthetic nitrogen-use efficiency of Pinus massoniana under in situ polluted stress. J Plant Res 124:663–673
Guisan A (2002) Semi-quantitative response models for predicting the spatial distribution of plant species. Predicting species occurrences: issues of accuracy and scale. Island Press, Covelo
Gvozdevaite A, Oliveras I, Domingues TF et al (2018) Leaf-level photosynthetic capacity dynamics in relation to soil and foliar nutrients along forest–savanna boundaries in Ghana and Brazil. Tree Physiol 38:1912–1925
Hikosaka K (2004) Interspecific difference in the photosynthesis–nitrogen relationship: patterns, physiological causes, and ecological importance. J Plant Res 117:481–494
Hikosaka K, Terashima I (1995) A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ 18:605–618
Katahata S, Naramoto M, Kakubari Y, Mukai Y (2005) Photosynthetic acclimation to dynamic changes in environmental conditions associated with deciduous overstory phenology in Daphniphyllum humile, an evergreen understory shrub. Tree Physiol 25:437–445
Katahata SI, Naramoto M, Kakubari Y, Mukai Y (2007) Photosynthetic capacity and nitrogen partitioning in foliage of the evergreen shrub Daphniphyllum humile along a natural light gradient. Tree Physiol 27:199–208
Kattge J, Knorr W, Raddatz T, Wirth C (2009) Quantifying photosynthetic capacity and its relationship to leaf nitrogen content for global-scale terrestrial biosphere models. Glob Chang Biol 15:976–991
Kitaoka S, Koike T (2004) Invasion of broad-leaf tree species into a larch plantation: seasonal light environment, photosynthesis and nitrogen allocation. Physiol Plant 121:604–611
Lawrence D (2018) CLM5 documentation. 309
LeBauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379
Long SP, Bernacchi CJ (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot 54:2393–2401
Maire V, Martre P, Kattge J et al (2012) The coordination of leaf photosynthesis links C and N fluxes in C3 plant species. PLoS ONE 7:e38345
McDowell SCL (2002) Photosynthetic characteristics of invasive and noninvasive species of Rubus (Rosaceae). Am J Bot 89:1431–1438
Meir P, Kruijt B, Broadmeadow M et al (2002) Acclimation of photosynthetic capacity to irradiance in tree canopies in relation to leaf nitrogen concentration and leaf mass per unit area. Plant Cell Environ 25:343–357
Niinemets Ü, Tenhunen JD (1997) A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ 20:845–866
Niinemets Ü, Bilger W, Kull O, Tenhunen JD (1998) Acclimation to high irradiance in temperate deciduous trees in the field: changes in xanthophyll cycle pool size and in photosynthetic capacity along a canopy light gradient. Plant Cell Environ 21:1205–1218
Niinemets Ü, Kull O, Tenhunen JD (2004) Within-canopy variation in the rate of development of photosynthetic capacity is proportional to integrated quantum flux density in temperate deciduous trees. Plant Cell Environ 27:293–313
Noda HM, Muraoka H, Nasahara KN et al (2015) Phenology of leaf morphological, photosynthetic, and nitrogen use characteristics of canopy trees in a cool-temperate deciduous broadleaf forest at Takayama, central Japan. Ecol Res 30:247–266
Novriyanti E, Watanabe M, Makoto K et al (2012) Photosynthetic nitrogen and water use efficiency of acacia and eucalypt seedlings as afforestation species. Photosynthetica 50:273–281
Oleson KW, Lawrence DM, Bonan GB et al (2013) Technical description of version 4.5 of the community land model (CLM). Natl Cent Atmos Res Boulder. https://doi.org/10.5065/D6RR1W7M
Onoda Y, Hikosaka K, Hirose T (2004) Allocation of nitrogen to cell walls decreases photosynthetic nitrogen-use efficiency. Funct Ecol 18:419–425
Onoda Y, Wright IJ, Evans JR et al (2017) Physiological and structural tradeoffs underlying the leaf economics spectrum. New Phytol 214:1447–1463
Poorter H, Evans JR (1998) Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 116:26–37
Quebbeman JA, Ramirez JA (2016) Optimal allocation of leaf-level nitrogen: implications for covariation of Vcmax and Jmax and photosynthetic downregulation. J Geophys Res Biogeosci 121:2464–2475
Rodríguez-Calcerrada J, Reich PB, Rosenqvist E et al (2008) Leaf physiological versus morphological acclimation to high-light exposure at different stages of foliar development in oak. Tree Physiol 28:761–771
Rogers A (2014) The use and misuse of Vc, max in earth system models. Photosynth Res 119:15–29
Rogers A, Serbin SP, Ely KS et al (2017) Terrestrial biosphere models underestimate photosynthetic capacity and CO2 assimilation in the Arctic. New Phytol 216:1090–1103
Song G, Wang Q, Jin J (2021) Exploring the instability of the relationship between maximum potential electron transport rate and maximum carboxylation rate in cool-temperate deciduous forests. Agric for Meteorol 308–309:108614
Sonobe R, Wang Q (2017) Hyperspectral indices for quantifying leaf chlorophyll concentrations performed differently with different leaf types in deciduous forests. Ecol Inform 37:1–9
Suzuki Y, Miyamoto T, Yoshizawa R et al (2009) Rubisco content and photosynthesis of leaves at different positions in transgenic rice with an overexpression of RBCS. Plant Cell Environ 32:417–427
Syphard AD, Franklin J (2009) Differences in spatial predictions among species distribution modeling methods vary with species traits and environmental predictors. Ecography 32:907–918
Takashima T, Hikosaka K, Hirose T (2004) Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ 27:1047–1054
Tian Y, Yuan H, Xie J, Zheng Y (2016) Shade tolerance and suitability of tree species for planting in rubber plantations. South for 78:11–18
Walker AP, Beckerman AP, Gu L et al (2014) The relationship of leaf photosynthetic traits— Vcmax and Jmax—to leaf nitrogen, leaf phosphorus, and specific leaf area: a meta-analysis and modeling study. Ecol Evol 4:3218–3235
Wang Q, IIo A, Tenhunen J, Kakubari Y (2008) Annual and seasonal variations in photosynthetic capacity of Fagus crenata along an elevation gradient in the Naeba Mountains, Japan. Tree Physiol 28:277–285
Wang S, Li Y, Ju W et al (2020) Estimation of leaf photosynthetic capacity from leaf chlorophyll content and leaf age in a subtropical evergreen coniferous plantation. J Geophys Res Biogeosci 125:e2019JG005020
Wilson KB, Baldocchi DD, Hanson PJ (2000) Spatial and seasonal variability of photosynthetic parameters and their relationship to leaf nitrogen in a deciduous forest. Tree Physiol 20:565–578
Wood SN (2017) Generalized additive models: an introduction with R. Chapman and Hall/CRC Press, Boca Raton
Wood SN (2020) Mixed GAM computation vehicle with automatic smoothness estimation. Version 1.8–33
Xu L, Baldocchi DD (2003) Seasonal trends in photosynthetic parameters and stomatal conductance of blue oak (Quercus douglasii) under prolonged summer drought and high temperature. Tree Physiol 23:865–877
Xu C, Fisher R, Wullschleger SD et al (2012) Toward a mechanistic modeling of nitrogen limitation on vegetation dynamics. PLoS ONE 7:e37914
Yee TW, Mackenzie M (1991) Generalized additive models in plant ecology. J Veg Sci 2:587–602
Yin X, Schapendonk AHCM, Struik PC (2019) Exploring the optimum nitrogen partitioning to predict the acclimation of C3 leaf photosynthesis to varying growth conditions. J Exp Bot 70:2435–2447
Zaehle S, Friend AD (2010) Carbon and nitrogen cycle dynamics in the O-CN land surface model: 1. Model description, site-scale evaluation, and sensitivity to parameter estimates. Global Biogeochem Cycles 24:GB1005
Zhong C, Jian SF, Huang J et al (2019) Trade-off of within-leaf nitrogen allocation between photosynthetic nitrogen-use efficiency and water deficit stress acclimation in rice (Oryza sativa L.). Plant Physiol Biochem 135:41–50
Ziehn T, Kattge J, Knorr W, Scholze M (2011) Improving the predictability of global CO2 assimilation rates under climate change. Geophys Res Lett 38:L10404
Zuur AF, Ieno EN, Walker NJ et al (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgements
We thank the members of the Laboratory of Macroecology and the Institute of Silviculture, Shizuoka University, for their support of both fieldwork and laboratory analyses.
Funding
This research was partially supported by the Japan Society for the Promotion of Science (JSPS) project (No. 21H02230).
Author information
Authors and Affiliations
Contributions
QW conceptualized the study. GS analyzed and interpreted the data. GS and QW wrote and compiled the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, G., Wang, Q. Seasonal dynamics of photosynthetic nitrogen content and partitioning in deciduous forests. Photosynth Res 156, 355–366 (2023). https://doi.org/10.1007/s11120-022-00992-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-022-00992-x