Abstract
Background
As the world grapples with increasing agricultural demands and unpredictable environmental stressors, there is a pressing need to improve plant resilience. Therefore, understanding the pioneering role of nanoparticles in alleviating plant stress is crucial for developing stress-resilient varieties to enhance food secure world. Nanoparticles have unique physical and chemical properties, and demonstrate their potential to enhance plant growth, nutrient utilization, and stress tolerance. This review delves into the mechanistic insights of nanoparticle-plant interactions, highlighting how these tiny particles can mitigate diverse stressors such as drought, salinity, and heavy metal toxicity. The action of different types of nanoparticles, including metal, carbon-based, and biogenic nanoparticles, are discussed in the context of their interaction with plant physiology and stress responses.
Aims
This article also explores the potential drawbacks and environmental implications of nanoparticle use, emphasizing the need for responsible and sustainable applications. Therefore, this study aimed to offer exciting possibilities for managing both biotic and abiotic stress in plant species, from improving water-use efficiency and stress resilience via nanotechnology.
Conclusions
Future research directions are suggested, focusing on nano-bioengineering and precision agriculture to create stress-resilient crops and enhance food security. Through the lens of interdisciplinary research, this paper underscores the significance of nanoparticles as innovative tools in the realm of agriculture, catalyzing a paradigm shift towards sustainable and stress-resilient farming systems.
Similar content being viewed by others
Introduction
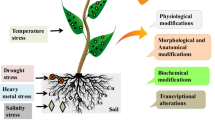
Nanotechnology offers a range of applications in agricultural systems, from improving nutrient utilization to pest and disease management, and has the potential to revolutionize sustainable agricultural practices (Gupta et al. 2023). Nanoparticles can interact at the molecular level with plant processes, potentially influencing growth, development, and response to stress conditions (Vijayakumar et al. 2022). Plants are subjected to abiotic stresses such as drought, salinity, and extreme temperatures. These conditions can induce oxidative stress, disrupt metabolic activities, and stunt growth. Nanoparticles (NPs), particularly metal oxide nanoparticles (e.g., iron oxide, zinc oxide, and copper oxide), can help mitigate these effects. For instance, iron oxide nanoparticles have been found to improve water use efficiency and chlorophyll content in wheat under drought stress (Manzoor et al. 2023; Nair and Chung 2014). Similarly, zinc oxide nanoparticles have been shown to increase tolerance to salinity stress in barley by enhancing antioxidant enzyme activity (Aslani et al. 2014; Singh et al. 2022). These examples demonstrate the potential of nanoparticles in managing abiotic stress in different plant species. Salt stress is a substantial abiotic stress factor that threatens the productivity and survival of a wide range of plant species globally (Al-Khayri et al. 2023b). High salinity can lead to ionic imbalance, osmotic stress, and oxidative damage, which collectively impact the physiological, biochemical, and molecular responses of plants (Mariyam et al. 2023; Munns and Tester 2008; Thabet et al. 2021b). NPs can trigger physiological and biochemical changes that increase the plant’s drought tolerance. For example, silver nanoparticles (AgNPs) were shown to enhance the antioxidant activity of wheat plants under drought stress, reducing oxidative damage and enhancing plant growth (El-Saadony et al. 2022). ZnO NPs can enhance the activities of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD) in plants under drought stress, helping to mitigate the harmful effects of reactive oxygen species (ROS) that are often produced under such stress conditions (Rehman et al. 2023). ZnO NPs can improve plant water use efficiency by modulating stomatal conductance and transpiration rate, which could help plants maintain their water balance under drought conditions (Seleiman et al. 2023). Developing strategies to enhance plant tolerance to salinity is, therefore, a critical goal for sustainable agriculture in saline-prone areas (Thabet and Alqudah 2023). Nanotechnology presents promising opportunities for addressing these challenges. NPs, due to their unique physicochemical properties, have been studied for their potential roles in alleviating several stress stimuli, including salinity and drought conditions (Saritha et al. 2022). The interaction of nanoparticles with plants can influence various aspects of plant physiology and biochemistry, from nutrient uptake to gene expression patterns. Moreover, NPs can also play a role in mitigating oxidative stress, a common consequence of high salinity (Khalid et al. 2022; Zanella et al. 2017). Unraveling these interactions would pave the way for innovative strategies to manage drought and salinity stress and enhance plant productivity. While the applications of nanotechnology in plant stress management are promising, it is crucial to understand potential ecological impacts (Upadhayay et al. 2023). Studies have found that some nanoparticles can have toxic effects on organisms, disrupt soil microbial communities, and bioaccumulation in food chains (Kookana et al. 2014; Maharramov et al. 2019). Research into the safe and sustainable application of nanotechnology in agricultural systems remains vital, with attention to both efficacies in improving plant health and productivity and minimizing potential environmental impact (Balusamy et al. 2023; Chaturvedi et al. 2006; Shang et al. 2019). Therefore, this study aimed to offer exciting possibilities for managing both biotic and abiotic stress in plant species, from improving water-use efficiency and stress resilience via nanotechnology. However, the potential environmental implications require careful consideration and continued research. This innovative technology presents a new frontier in sustainable agricultural practices, potentially transforming how we approach plant stress management.
Nanoparticle (NP) classes
Nanoparticles (NPs) are particles that have at least one dimension less than 100 nm. NPs exist in various forms and are classified based on their composition, properties, and potential applications (Abdal Dayem et al. 2017) as shown in Fig. 1. Based on their composition, NPs are generally placed into three classes: organic, carbon-based, and inorganic.
Organic NPs
The NPs in this class are proteins, polysaccharides, lipids, polymers, or other organic substances (Pan and Zhong 2016). The most common examples of this class are dendrimers, liposomes, micelles, and protein complexes like ferritin. These biodegradable, non-toxic NPs can contain a hollow core, like liposomes. They are also produced by non-covalent intermolecular interactions, making them more labile and allowing for body clearance (Ng and Zheng 2015). Organic NPs’ potential applications depend on their composition, surface shape, stability, carrying capacity, etc. Organic NPs are typically used in cancer medication and targeted drug delivery (Gujrati et al. 2014).
Carbon-based NPs
This class includes carbon-only NPs. Carbon quantum dots, fullerenes, and carbon black NPs are famous examples. Fullerenes are symmetrical closed-cage carbon compounds. The shape of C60 fullerenes is a soccer ball, while C70 and C540 fullerenes have also been described (Long et al. 2013). Grape-shaped carbon black NPs are strongly fused spherical particles. Carbon quantum dots are discrete, quasi-spherical carbon NPs under 10 nm (Lu et al. 2016). Carbon-based NPs combine sp2-hybridized carbon bonds with nanoscale physicochemical characteristics. Due to their unique electrical conductivity, high strength, electron affinity, optical, thermal, and sorption properties, carbon-based NPs are used in drug delivery, energy storage, bioimaging, photovoltaic devices, and environmental sensing to monitor microbial ecology or detect pathogens (Mauter and Elimelech 2008). Complex carbon-based NPs include nanodiamonds and carbon nanoparticles. Their low toxicity and biocompatibility make them useful in medication administration and tissue engineering (Mochalin et al. 2012).
Inorganic NPs
NPs without carbon or organic components are in this class. These NPs are usually metal, ceramic, or semiconductor. Monometallic, bimetallic, or polymetallic metal NPs are formed using metal precursors (Nascimento et al. 2018). Bimetallic NPs can be alloyed or core–shell. Due to localized surface plasmon resonance, these NPs have unique optical and electrical properties (Toshima and Yonezawa 1998). Additionally, some metal NPs have unique thermal, magnetic, and biological features. Therefore, it renders them crucial materials for developing nanodevices for physical, chemical, biological, biomedical, and pharmaceutical uses (Fedlheim and Foss 2001; Khan et al. 2019). Today, size-, shape-, and facet-controlled metal NP production is essential for advanced materials. Semiconductor materials have metal-non-metal characteristics. These NPs have broad bandgaps and differ from bulk semiconductor materials in their characteristics when tuned (Dreaden et al. 2012). Thus, these NPs are significant in photocatalysis, optics, and electronics (Sun et al. 2000). Ceramic NPs are carbonates, carbides, phosphates, and metal and metalloid oxides including titanium and calcium (Thomas et al. 2015). They can be amorphous, polycrystalline, dense, porous, or hollow and are produced by heating and cooling (Khan et al. 2019). Their stability and load capacity make them popular in biomedical applications (Moreno-Vega et al. 2012). They also work on catalysis, dye degradation, photonics, and optoelectronics (Thomas et al. 2015).
Synthesis and characterization of metal oxide nanoparticles
Metal oxide nanoparticles have gained significant interest in various fields due to their unique physical and chemical properties, which are distinct from their bulk counterparts. Their small size provides a large surface area-to-volume ratio, making them particularly useful in applications such as catalysis, electronics, and medicine. The synthesis and characterization of these nanoparticles are crucial steps in fully utilizing their potential as shown in Fig. 2.
Synthesis of metal oxide nanoparticles
There are several methods to synthesize metal oxide nanoparticles, including physical, chemical, and green methods (Singh et al. 2018).
Physical methods include ball milling, physical vapor deposition, laser ablation, and others. While these methods can provide good control over particle size, they often require high energy inputs and specialized equipment. Here, we will discuss the laser ablation method: The process of laser ablation involves the utilization of high-power laser pulses in conjunction with a high vacuum system equipped with inert gas capabilities. This process requires the presence of a target material, a laser beam, and a cooled surface, sometimes referred to as a substrate. The utilization of ultraviolet (UV) lasers, such as excited monomer lasers, is commonly necessary in this process due to the tendency of some materials’ substrates to reflect wavelengths such as infrared (IR) or visible light. The strong laser beam evaporates the substrate’s atoms, causing reactive gasses to collide and form clusters on the cooled surface (Fig. 2). Particle size and dispersion require gas pressure. Evaporating and combining two vaporized materials in inert gas forms alloys or compounds. This method can create unusual component phases. This approach produces Y2O3, Gd2O3, Y3Al5O12, Al2O3, and YAlO3 NPs with typical diameters of 2–4.5 nm and limited size distribution (Amans et al. 2011). Laser ablation is a one-spot method for producing MONPs and hydroxides and preparing their layers, unlike some chemical methods. This method produced 2–6 nm-diameter TiO2 and SnO2 NPs by Sasaki et al. (2006).
Chemical methods are the most commonly used and include methods such as sol–gel synthesis, hydrothermal synthesis, co-precipitation, and micro-emulsion techniques. Sol–gel synthesis, for instance, involves the reaction of a metal alkoxide precursor in a solvent, followed by gelation, aging, drying, and heat treatment to form the metal oxide nanoparticles (Bokov et al. 2021). Chemical methods generally provide good control over particle size and morphology but can involve hazardous chemicals and generate waste.
Green synthesis methods, on the other hand, use biological entities like plant extracts or microbes to reduce metal ions to form nanoparticles. This method is environmentally friendly and often does not require high temperatures or pressures (Patel 2022). However, it can be challenging to control the size and shape of the nanoparticles using green synthesis methods (Kuppusamy et al. 2016).
Characterization of metal oxide nanoparticles
Characterizing metal oxide nanoparticles is crucial to understand their properties and behavior. Various techniques can be employed for this purpose:
Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM) are used to determine the shape and size of the nanoparticles (Goldstein et al. 2003).
X-ray Diffraction (XRD) is used to identify the crystalline phases present in the nanoparticle sample, providing information on the crystal structure and size of the nanoparticles (Pecharsky and Zavalij n.d.).
Fourier Transform Infrared Spectroscopy (FTIR) can be used to identify the functional groups present on the surface of the nanoparticles, giving information about possible surface modifications (Stuart 2021).
Dynamic Light Scattering (DLS) and Zeta Potential measurements are used to determine the hydrodynamic size of the nanoparticles in solution and their surface charge, respectively, which are important for understanding the stability of nanoparticle suspensions (Berne and Pecora 2000). Overall, the synthesis and characterization of metal oxide nanoparticles are crucial steps in leveraging their unique properties for various applications.
Nanoparticle uptake in plants
NPs have unique physical and chemical properties that make them useful for a variety of applications. The interaction between nanoparticles and plants, including their uptake, is an area of active research. Different types of nanoparticles may interact differently with plant tissues and have varied modes of uptake. Here, we will discuss several common types of nanoparticles and their potential uptake mechanisms.
The uptake of nanoparticles by plants can happen through several mechanisms, largely depending on the size, shape, and surface chemistry of the nanoparticles, as well as the plant species and environmental conditions. This is the primary route of nanoparticle uptake in plants. NPs present in the soil solution can enter the plant roots through the apoplast (non-living spaces in the plant), symplast (living parts of the plant), or endocytosis (Rico et al. 2011). NPs can also enter plants through the stomata, small openings on the leaf surface. This pathway is especially relevant for airborne nanoparticles (Vittori Antisari et al. 2015). The plant cuticle, a waxy layer on the aerial parts of the plant, can also be a site of nanoparticle uptake, particularly for lipid-soluble nanoparticles (Raliya et al. 2015). There’s evidence suggesting that nanoparticles can exploit natural plant uptake mechanisms, such as ion channels and transporters, although the specifics can vary depending on the size, charge, and composition of the nanoparticles. reported several factors affecting the uptake of NPs. For instance, the physicochemical properties of nanoparticles such as size, shape, surface charge, and coating significantly influence their uptake by plants. For example, smaller nanoparticles are generally more readily absorbed due to their higher surface-area-to-volume ratio and increased reactivity. Environmental conditions, including pH, temperature, and the presence of other ions or organic matter, can also affect nanoparticle uptake, often by altering nanoparticle stability or the physiology of the plant’s uptake systems (Joudeh and Linke 2022). Once inside the plant, nanoparticles can be translocated from the roots to other parts of the plant via the xylem and phloem, though the efficiency of this process depends on the nanoparticles’ properties. Studies have shown that nanoparticles can accumulate in various plant tissues, including leaves, stems, and fruits/seeds, which has implications for food safety and environmental health. The degree of accumulation can be influenced by the duration of exposure and the metabolic processes of the plant species (Wang et al. 2023). Moreover, the uptake of nanoparticles can trigger various biological responses in plants, ranging from nutrient use efficiency to stress tolerance and even changes in gene expression (Wang et al. 2023). However, it can also cause toxicity, leading to effects like reduced growth, oxidative stress, and alterations in physiology and metabolism, particularly at higher concentrations (Azameti and Imoro 2023). Recent studies have used advanced imaging techniques, such as electron microscopy and synchrotron radiation-based analysis, to trace the biodistribution of nanoparticles within plant tissues and cells (Zhao et al. 2022). These techniques help in understanding the fate of nanoparticles inside plants, including any potential biotransformation (chemical changes that nanoparticles might undergo inside the plant) (Zhao et al. 2022). Moreover, as we gain more insights into these interactions, there’s a growing need for developing standardized methodologies for studying nanoparticle uptake, translocation, and accumulation in plants, to ensure data comparability and reliability across different studies. The implications for food safety, environmental implications, and regulatory policies also require careful consideration.
Mitigating plant stress: the promising role of nanoparticles in agro-biotechnology
Nanotechnology has the potential to provide new strategies to combat abiotic stresses like drought and salinity that drastically affect plant growth and productivity. Water scarcity or drought is one of the major abiotic stresses affecting plant growth and development. NPs can play a significant role in mitigating drought stress. Certain types of nanoparticles, such as hydrogel nanoparticles, can retain a large amount of water and slowly release it to the plants, thereby enhancing plant growth under water-limited conditions (Nair and Chung 2014). NPs can trigger physiological and biochemical changes that increase the plant’s drought tolerance. For example, silver nanoparticles (AgNPs) were shown to enhance the antioxidant activity of wheat plants under drought stress, reducing oxidative damage and enhancing plant growth (El-Saadony et al. 2022). ZnO NPs can enhance the activities of antioxidant enzymes such as catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD) in plants under drought stress, helping to mitigate the harmful effects of reactive oxygen species (ROS) that are often produced under such stress conditions (Rehman et al. 2023). ZnO NPs can improve plant water use efficiency by modulating stomatal conductance and transpiration rate, which could help plants maintain their water balance under drought conditions (Seleiman et al. 2023). The use of TiO2 NPs can help plants tolerate drought stress via several mechanisms such as photosynthetic efficiency and antioxidant activation. TiO2 NPs have been found to improve the efficiency of photosynthesis under drought stress by increasing chlorophyll content, leading to enhanced plant growth and productivity (Ramadan et al. 2022; Thabet et al. 2021a). Like other types of nanoparticles, TiO2 NPs can enhance the activity of antioxidant enzymes in plants under drought stress. This enhanced antioxidant activity can protect the plant cells from oxidative damage caused by ROS (Cevik 2023; Samanta et al. 2024). AgNPs can lead to physiological changes in plants that help them survive under water-limited conditions. For example, they can improve plant water use efficiency and modify stomatal conductance to reduce water loss (Yan and Chen 2019). AgNPs can enhance the activity of antioxidant enzymes in plants under drought stress, which helps to alleviate the oxidative damage caused by the increased production of ROS (Al-Khayri et al. 2023b; Khan et al. 2020). In general, the use of nanoparticles can potentially provide innovative solutions to combat abiotic stresses.
Salinity stress can lead to the accumulation of toxic ions in the plant, water imbalance, and nutrient deficiency, and can impair various physiological processes. Certain nanoparticles can induce changes in the plants that increase their salt tolerance (Etesami et al. 2021). For instance, ZnO NPs were shown to promote the growth of maize seedlings under salt stress conditions by enhancing the activities of catalase, peroxidase, and superoxide dismutase, which helped in scavenging the harmful ROS produced due to salt stress (Faizan et al. 2021). NPs can also deliver essential nutrients to the plant in a controlled manner, helping to counteract nutrient deficiencies caused by high salt concentrations. ZnO NPs can potentially help alleviate these adverse effects by enhancing salt tolerance and regulating ion homeostasis. The application of ZnO NPs has been found to promote plant growth under saline conditions by enhancing antioxidant enzyme activities, reducing ROS levels, and alleviating lipid peroxidation caused by high salt levels (Abdel Latef et al. 2017). ZnO NPs can potentially regulate ion homeostasis in plants exposed to high salinity, thereby alleviating the toxic effects of sodium ions and enhancing the uptake of beneficial ions (Yasmin et al. 2021). TiO2 NPs can improve the uptake and utilization of nutrients in plants growing in saline conditions. This can help counteract the nutrient imbalances caused by high salt concentrations (Abdel Latef et al. 2018). A recent study reported the crucial role of the foliar application of selenium nanoparticles in barley under saline soil (Thabet and Alqudah 2023). The promising roles of TiO2 NPs in mitigating both drought and salinity stress are attributed to their potential long-term effects on plant health, potential toxicity, and overall environmental impact (Thabet et al. 2021a). Moreover, AgNPs can potentially mitigate these effects. The application of AgNPs can promote plant growth under saline conditions by enhancing antioxidant enzyme activities, reducing ROS levels, and improving nutrient uptake (Al-Khayri et al. 2023b). AgNPs can help alleviate the ionic imbalance caused by high salt levels by modulating the uptake and accumulation of ions in plant cells (Abasi et al. 2022). Ultimately, plant responses to drought and salinity stress involve numerous complex physiological and molecular processes, which are often closely related to membrane stability and plant-water relationships as depicted in Fig. 3. NPs have been increasingly studied for their potential roles in enhancing plant tolerance to these stresses. Furthermore, certain NPs can improve nutrient uptake in plants under stress conditions. For instance, nano-fertilizers have a high surface area-to-volume ratio, enhancing their solubility and availability to plants compared to conventional fertilizers (Azameti and Imoro 2023). This property is particularly beneficial under stress conditions that typically reduce nutrient availability or uptake, helping plants maintain their metabolic activities and growth even under adverse conditions (Abdel-Hakim et al. 2023). Moreover, NPs, particularly biochar-based or carbon-based, can adsorb heavy metals in the soil, reducing their uptake by plants. This action helps in mitigating the toxic effects of heavy metal stress (Chausali et al. 2021). Additionally, certain nanoparticles can induce the expression of metallothioneins, proteins that bind and sequester heavy metals, thus reducing their toxicity to plant cells (Rai et al. 2023). While nanoparticles hold promising potential for enhancing plant stress tolerance, it’s essential to approach their use cautiously. The long-term effects and ecological impacts of nanoparticle exposure in agriculture remain subjects of extensive research, and their interactions with plants and ecosystems can be complex and still not fully understood. Further studies, particularly at the genomic, proteomic, and metabolomic levels, are required to unravel the detailed mechanisms of how different types of nanoparticles interact with plant systems under various environmental stress conditions.
Genetic network models regulating source-to-sink interaction underlying sugar-metabolism and their role in spike/spikelet development. Suc: Sucrose, Fru: Fructose, Glc: Glucose, TPS: Trehalose Phosphate Synthase, TPP: Trehalose Phosphate Phosphatase, Tre: Trehalose, UDP: Uridine Diphosphate, G6P: Glucose-6-Phosphate, HXK: Hexokinase
Nanoparticles improve membrane stability, plant-water relationships, and nutrient delivery
Improvement of membrane stability
Mitigating oxidative damage is one of the major impacts of drought and salinity stress due to the overproduction of ROS, which can cause oxidative damage to cell membranes. Nanoparticles, such as AgNPs and TiO2 NPs, can enhance the activity of antioxidant enzymes, helping to mitigate oxidative damage and maintain membrane stability (Al-Khayri et al. 2023b). Under salinity stress, excess sodium ions can disrupt ionic balance and harm the cell membrane. Certain nanoparticles, like silicon dioxide nanoparticles (SiO2 NPs), can modulate the activity of ion channels and transporters, helping to maintain ionic balance and protect the membrane (Mahmoud et al. 2020).
Enhancement of plant-water relationships
The role of nanoparticles in enhancing plant-water relationships is pivotal, especially in an era where water scarcity and environmental challenges threaten sustainable agricultural practices (Rehman et al. 2024). Plant-water relationships involve the absorption, transport, and utilization of water by plants, which are critical processes for plant growth, photosynthesis, and overall productivity (Rasheed et al. 2022a). Nanotechnology offers innovative approaches to improve water efficiency and resilience in plants, addressing several aspects:
NPs, due to their minute size and high surface area, can alter the soil’s physical properties to enhance its water-holding capacity (Khan et al. 2019). Certain NPs can carry and release nutrients in a controlled manner, ensuring that plants receive a steady supply of essential nutrients without excessive water (El-Saadony et al. 2022). Nano-fertilizers require less water compared to traditional fertilizers, as they minimize nutrient leaching and runoff, thereby promoting efficient water use and preventing groundwater contamination. (Yadav et al. 2023a) At the molecular level, NPs can interact with plant cells, potentially altering the expression of genes involved in water stress responses. For instance, nanoparticle-mediated delivery of genetic material or hormones could help plants maintain better cell turgor pressure and osmotic balance, enhancing their tolerance to drought or salinity by regulating stomatal opening and closing, thus optimizing water usage (Al-Khayri et al. 2023a). Nanopesticides and nanocarriers for biocontrol agents can target specific pests and diseases with precision, reducing the need for broad-spectrum chemicals and excessive irrigation associated with traditional pesticide application (Chaud et al. 2021). Healthier plants are more efficient in their water usage and can maintain optimal water relationships (Yadav et al. 2023b). For monitoring plant water status, nanosensors can be used to monitor plant water status in real-time, allowing for precise irrigation when it’s most needed. These sensors can detect minute changes in soil moisture, humidity, and plant water stress, ensuring that plants receive the right amount of water at the right time, thus preventing both water scarcity and waterlogging conditions (Li et al. 2022a). Certain NPs can also influence the light absorption efficiency of photosynthetic pigments, potentially enabling plants to produce more biomass with the same amount of water or maintain productivity under reduced water availability (Nayeri et al. 2023). Moreover, NPs can be used for the remediation of polluted water sources, making them safe for irrigation. For instance, they can bind to heavy metals or degrade organic pollutants, thereby preventing these contaminants from entering the plant system and the broader ecosystem (Okeke et al. 2023). In conclusion, nanoparticles hold significant promise in optimizing plant-water relationships is critical for sustainable agriculture and food security. However, this emerging field requires cautious optimism, with policies and research geared towards maximizing benefits while mitigating potential risks.
Improvement nutrient delivery
Under stress conditions, plants often experience significant challenges in nutrient uptake due to the decreased availability and mobility of nutrients, as well as changes in soil properties and plant physiology (Agnihotri et al. 2018). NPs, due to their unique properties, can play a pivotal role in improving nutrient uptake under these stressful conditions (Nongbet et al. 2022). Also, NPs, due to their small size, high surface area, and ability to carry and release nutrients in a controlled manner, can improve nutrient delivery to plants (Elemike et al. 2019). Nano fertilizers, which contain essential nutrients encapsulated in nanoparticles, can enhance the availability and uptake of nutrients by plants. For example, Nano-fertilizers containing nitrogen, phosphorus, potassium, or micronutrients can significantly improve plant nutrition under stress conditions (Nongbet et al. 2022). Under salinity stress, high salt concentrations can interfere with the absorption of essential nutrients by creating an ionic imbalance. Certain types of nanoparticles, such as silicon dioxide (SiO2) nanoparticles, have been shown to help mitigate this ionic imbalance, thereby improving nutrient uptake (Etesami et al. 2021; Tripathi et al. 2017). Nanoparticles can regulate plant physiological processes, leading to enhanced nutrient uptake. For instance, studies have shown that certain nanoparticles can upregulate the expression of nutrient transporters, thus improving nutrient acquisition under stressful conditions (Junedi et al. 2023; Tan et al. 2017). Some nanoparticles can alter soil properties to improve nutrient availability. For example, biochar nanoparticles can increase soil water-holding capacity and nutrient retention, thus enhancing nutrient availability under drought and salinity stress (Liu and Lal 2015).
The role of nanoparticles in protecting photosynthetic apparatus
The photosynthetic apparatus, including chloroplasts, photosynthetic pigments, and photosystems, is among the most sensitive targets of drought and salinity stress (Sherin et al. 2022). Under such stress conditions, ROS production increases, leading to oxidative stress that damages the photosynthetic machinery and decreases photosynthesis (Foyer 2018). NPs can help to protect the photosynthetic apparatus and improve photosynthesis under these stressful conditions (Dilnawaz et al. 2023; Kataria et al. 2019). Some nanoparticles, such as cerium oxide (CeO2) and TiO2 nanoparticles, have been shown to protect the photosynthetic machinery from oxidative damage. They can act as ROS scavengers, reducing oxidative stress and preserving the integrity and function of chloroplasts and photosystems (Chen et al. 2015; Kumar et al. 2023; Singh and Lee 2016). NPs can improve the synthesis of photosynthetic pigments, such as chlorophyll, which are essential for photosynthesis. For example, ZnO and Ag nanoparticles can enhance chlorophyll content under drought and salinity stress, thus improving photosynthesis (Rizwan et al. 2017; Vannini et al. 2014). Certain NPs have antioxidant properties, allowing them to scavenge ROS generated under stress conditions (Choudhary et al. 2024). For example, cerium oxide nanoparticles and manganese nanoparticles mimic the action of superoxide dismutase, a natural antioxidant enzyme in plants, neutralizing ROS and thereby protecting the photosynthetic apparatus from oxidative damage (Padmanaban et al. 2023). NPs can enhance the uptake of nutrients by modifying root characteristics or influencing soil fertility, thereby promoting plant growth and overall health (Al-Mamun et al. 2021). Certain nanoparticles, such as zinc oxide and TiO2, can act as UV filters. When applied to plants, these nanoparticles can scatter or absorb harmful UV rays, protecting the photosynthetic apparatus from potential UV-induced damage (Liang et al. 2022). Some nanoparticles can confer thermo-protective effects on plants, helping them survive in extreme temperatures. This protection ensures the integrity of the photosynthetic apparatus is maintained, even under heat stress conditions (Karumannil et al. 2023). NPs have shown promise in enhancing water use efficiency in plants. For instance, hydrogel nanoparticles can absorb water and release it slowly, allowing plants to access water over extended periods, which is especially critical during drought conditions. By maintaining proper hydration, the photosynthetic apparatus can function more effectively (Patra et al. 2022). Moreover, NPs can modulate the expression of genes related to photosynthesis, thus enhancing photosynthetic efficiency (Rasheed et al. 2022b). For instance, carbon-based nanoparticles have been shown to upregulate genes involved in chloroplast development and photosynthesis under stress conditions (Chandrashekar et al. 2023). Also, NPs can enhance the stability of the photosynthetic process under stress conditions. For instance, silica nanoparticles have been found to stabilize photosystem II (PSII) and enhance photosynthetic performance under salinity stress (Qi et al. 2013; Rasheed et al. 2022a). However, it’s crucial to note that while nanoparticles offer these potential benefits, their interaction with plants and ecosystems must be thoroughly understood to prevent possible negative effects. There are concerns regarding toxicity and bioaccumulation, which could adversely affect plant health, soil quality, and the broader ecosystem. Therefore, while nanoparticles present a promising tool for protecting the photosynthetic apparatus, their application must be approached with caution, comprehensive understanding, and regulatory oversight.
Maintenance of hormonal crosstalk
Plant hormones such as abscisic acid (ABA), salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) play key roles in plant responses to drought and salinity stress. These hormones often interact with each other, a phenomenon known as hormonal crosstalk, to regulate plant stress responses. Nanoparticles can modulate this hormonal crosstalk to enhance plant tolerance to stress. Alabdallah and Hasan (2021) reported that AgNPs can increase the levels of ABA, a hormone that plays a crucial role in plant responses to drought and salinity stress. AgNPs can induce a stress-like response in plants, even in the absence of typical environmental stressors. The nanoparticles are often perceived by plants as foreign elements, which can trigger a cascade of reactions within the plant’s internal defense system. This stress perception is known to induce the synthesis of ABA, as the hormone acts as a signaling molecule in various stress responses, including those initiated by AgNPs (Yan and Chen 2019). One of the well-known roles of ABA is the regulation of stomatal closure to reduce water loss in times of drought stress. Studies have shown that AgNPs can cause stomatal closure, and it’s suggested that this may be due to the increased levels of ABA. When AgNPs are absorbed by plant roots or taken up by foliage, they may stimulate the plant’s signaling pathway to increase ABA synthesis, leading to reduced transpiration through stomatal closure (Bharath et al. 2021). Elevated ROS levels are a signal for stress conditions, and plants often respond by increasing the production of antioxidants and signaling molecules like ABA to mitigate the stress conditions (Li et al. 2022b). Thus, AgNPs can indirectly result in increased ABA levels through enhanced ROS production (Abasi et al. 2022). Some studies have found that AgNPs can influence seed germination rates and overall plant growth. As ABA is a regulator of seed dormancy and germination, AgNPs may impact these processes through the modulation of ABA levels (Nadarajah 2020; Noga et al. 2023). While the relationship between AgNPs and increased ABA levels in plants highlights the potential of nanotechnology in the field of agriculture, such as in the development of new stress-resistance cultivars or management practices, it’s also important to note the necessity for detailed studies on potential toxicity and environmental impacts of AgNPs. Further research is needed to fully understand the underlying mechanisms of how AgNPs interact with plant hormonal pathways, including those involving ABA (Tariq et al. 2022). Therefore, the use of nanoparticles in the maintenance of hormonal crosstalk is important for improving plant growth and development under stressful environmental conditions.
Nanoparticles improve phenolic compounds accumulation
Plants often increase the production of phenolic compounds, which are secondary metabolites that play significant roles in stress responses due to their antioxidant, UV-absorbing, and signaling properties (Lin et al. 2016). NPs can enhance the accumulation of these phenolic compounds under stress conditions, thereby improving plant stress tolerance. NPs can stimulate the biosynthesis of phenolic compounds (Rasheed et al. 2022c). For example, recent studies have reported that the application of AgNPs and ZnONPs can enhance the production of phenolic compounds in plants under drought and salinity stress (Kim et al. 2024b; Kumari et al. 2022). NPs can regulate the metabolic pathways related to phenolic compounds (Bontempo et al. 2023). This can be achieved through the modulation of the activities of key enzymes in these pathways, such as phenylalanine ammonia-lyase (PAL), which catalyzes the first step in the biosynthesis of phenolic compounds. Studies have demonstrated that nanoparticles like nano-TiO2 can enhance the activity of PAL, thereby promoting phenolic metabolism (Shah et al. 2021). Furthermore, NPs can improve the storage and mobilization of phenolic compounds, which can be crucial under stress conditions (Geremew et al. 2023). For instance, Yin et al. (2012) detected that SiO2 nanoparticles have been shown to improve the compartmentalization of phenolic compounds in vacuoles, which can protect plant cells from the potential toxicity of these compounds and ensure their availability when needed.
Nanoparticles enhance sugar metabolism
Nanotechnology, the manipulation of matter at the atomic and molecular scale, has shown remarkable potential in various fields, including biomedicine, electronics, and agriculture. Recent research has revealed that nanoparticles can play a pivotal role in modulating plant physiological processes, including sugar metabolism, especially under stress conditions (Bayda et al. 2019). Here, we delve into the role of nanoparticles in enhancing sugar metabolism in plants under stress responses. Before delving into nanoparticles, it’s essential to understand the relationship between stress and sugar metabolism in plants. Furthermore, plants, when subjected to various abiotic stresses (e.g., drought, salinity, temperature extremes), show alterations in their primary metabolism, particularly sugar metabolism (Saba et al. 2023a). Sugar molecules, apart from being metabolic intermediates, also act as signaling molecules and osmoprotectants, helping plants cope with stress conditions (Sharma et al. 2019). Therefore, modulating sugar metabolism can potentially enhance plant resilience to stressful environments (Fig. 3). Certain nanoparticles, when applied to plants, can interact directly with the enzymes and intermediates involved in sugar metabolism pathways. This interaction can either promote or inhibit specific reactions, leading to an increased accumulation of sugars, which can act as osmoprotectants under stress conditions (Hao et al. 2021). NPs can also influence the expression of genes related to sugar metabolism. This gene-level interference can amplify or diminish the synthesis of enzymes crucial for sugar metabolic pathways, thus allowing plants to adjust their metabolic processes according to environmental cues (Selvakesavan et al. 2023). Various nanoparticles have been investigated for their role in modulating plant sugar metabolism. For instance, Exposure to AgNPs has been reported to increase sugar content in some plants (Kim et al. 2024a). This could be due to the oxidative stress generated by AgNPs, leading plants to accumulate more sugars as a protective mechanism (Abasi et al. 2022). Silicon, a beneficial element for plants, when delivered in nanoparticulate form, can bolster sugar metabolism, especially under drought stress. It can enhance the enzymatic activity and facilitate better sugar regulation in plants (Irfan et al. 2023). In conclusion, nanoparticles hold potential in modulating sugar metabolism in plants, especially under stress conditions. This can be a novel avenue for enhancing crop productivity in increasingly challenging environmental conditions.
Nanoparticles improve the expression of stress-responsive genes
NPs can play a significant role in enhancing the expression of stress-responsive genes under drought and salinity stress. These genes play a critical role in plant responses to stress and can lead to changes in plant physiology, metabolism, and growth that enhance stress tolerance (Prajapati et al. 2023). For example, the application of ZnONPs has been detected to upregulate the expression of genes involved in salt stress responses in plants (Sheteiwy et al. 2021; Spanò et al. 2020). These genes encode proteins involved in a variety of processes, including ion transport, signaling, and ROS detoxification. Transcription factors are proteins that control the expression of other genes and play a key role in plant responses to stress (Trono and Pecchioni 2022). NPs can modulate the activity of these transcription factors, thereby influencing the expression of stress-responsive genes. Gomaa et al. (2021) reported that AgNPs can modulate the activity of transcription factors involved in stress responses, leading to enhanced plant stress tolerance.
Toxicity of nanoparticles in agriculture
Nanotechnology has tremendous potential in agriculture due to its ability to enhance nutrient absorption, pest control, and disease management, and improve the overall sustainability of agricultural practices (Prasad et al. 2017). However, the introduction of nanoparticles into the ecosystem could have unintended consequences, leading to potential toxicity and hazards. One major concern is that nanoparticles used in agriculture (for instance, nanopesticides, nanofertilizers, or nano-enabled water treatment systems) might accumulate in the environment (Iavicoli et al. 2017; Mohammadi et al. 2023). Due to their small size, nanoparticles can easily be transported by water or wind, leading to contamination of water sources, soil, or unintended ecosystems (Ray et al. 2009). Their high reactivity and surface area-to-volume ratio might also result in interactions with native species or bioaccumulation in the food chain, potentially disrupting ecosystem balances (Iavicoli et al. 2017). NPs can adversely affect soil health by altering the microbial community structure. The soil microbiota plays a crucial role in nutrient cycling, organic matter decomposition, and plant health (Zhu et al. 2022). NPs might be toxic to certain microorganisms, potentially disrupting these processes and affecting plant growth and soil health (Khanna et al. 2021). Although some nanoparticles are designed to be beneficial to plants, they can become toxic at certain concentrations or sizes due to the enhanced reactivity. This can lead to phytotoxicity, affecting plant growth, seed germination rates, and crop yield (Shelar et al. 2023). In some cases, nanoparticles can induce oxidative stress in plants, damaging cellular structures and inhibiting normal plant functions (Budhani et al. 2019). Since agricultural products are part of the human food supply, there’s a risk that nanoparticles used in agriculture might accumulate in edible parts of plants or in animal tissues, leading to potential health risks if consumed (Mittal et al. 2020). The long-term health effects of consuming nanoparticles are not entirely understood and are a subject of ongoing research (He et al. 2019). There’s currently a lack of comprehensive regulation concerning the use of nanotechnology in agriculture. This is partially due to the relatively nascent state of the field, but also because of the complex behavior of nanoparticles, which makes them difficult to categorize and assess under traditional regulatory frameworks (Kumari et al. 2023). Given these potential risks, it’s crucial to approach the use of nanotechnology in agriculture with caution. Comprehensive risk assessments, clear regulations, and thorough research into the long-term implications of nanomaterial exposure on environmental and human health are essential. Responsible nanotechnology use in agriculture should balance the potential benefits with a cautious approach to avoid unforeseen negative impacts.
Nanoparticles improve growth, yield, and quality
NPs have shown great potential in improving the growth, yield, and quality of plants under drought and salinity stress conditions (Azameti and Imoro 2023). Their unique properties and interactions with plants can lead to beneficial effects on plant physiology, metabolism, and overall performance. NPs can promote plant growth under stress challenges by improving nutrient uptake, regulating hormone levels, and mitigating stress-induced damage (Hayat et al. 2023). The application of SiNPs has been shown to enhance root development, increase shoot biomass, and improve overall plant growth under drought and salinity stress (Mukarram et al. 2022). Similarly, ZnONPs have been found to enhance root and shoot growth, leading to improved plant performance under stress conditions (Rizwan et al. 2019). In addition, NPs can positively influence crop yield under drought and salinity stress. By improving nutrient availability, photosynthetic efficiency, and stress tolerance, nanoparticles can enhance the productivity of plants (Alharbi et al. 2023; Khan et al. 2022). Saba et al. (2023b) reported increased yield in crops such as wheat, rice, and maize when treated with nanoparticles under stress conditions. These yield improvements can be attributed to the nanoparticles’ ability to alleviate stress-induced growth constraints and enhance plant physiological processes. NPs have the potential to improve the nutritional quality of crops grown under drought and salinity stress (Al-Khayri et al. 2023b). They can enhance the accumulation of essential nutrients, vitamins, and antioxidants, which are important for human health. For instance, the application of SeNPs has been reported to increase the selenium content in plants, thus improving their nutritional value (Bano et al. 2021; Thabet and Alqudah 2023). Additionally, NPs can enhance the synthesis of bioactive compounds and secondary metabolites, contributing to improved quality traits in crops. Nanoparticles can regulate various physiological processes in plants, enabling them to better cope with drought and salinity stress (Selvakesavan et al. 2023). While NPs show promise in improving growth, yield, and quality under drought and salinity stress, it is important to consider factors such as nanoparticle dosage, application methods, and potential environmental impacts.
NPs bring ultra-structural changes to induce stress tolerance
NPs have shown the ability to induce ultra-structural changes in plants, which can contribute to improved drought and salinity tolerance. These structural modifications at the cellular and subcellular levels enhance the plant’s ability to withstand water scarcity and high salt concentrations (Raza et al. 2023). NPs can play a vital role in protecting the integrity of the cell membrane under environmental stress conditions. By interacting with the lipid bilayer, nanoparticles can prevent the loss of membrane integrity, reduce membrane permeability, and enhance the overall stability of the membrane structure (Wang et al. 2017). This protection helps to maintain cellular homeostasis and prevent the entry of harmful ions, thereby improving stress tolerance (Bora et al. 2022). NPs can influence stomatal behavior, which is crucial for controlling water loss through transpiration. They can regulate the opening and closing of stomata, reducing transpiration rates and conserving water under drought and salinity stress conditions (Seleiman et al. 2021). This regulation helps to maintain plant hydration levels and enhance water use efficiency (Cominelli et al. 2010). Also, NPs can protect cellular organelles, such as chloroplasts and mitochondria, against stress-induced damage (Kumar et al. 2024). These organelles play essential roles in energy production and stress responses. Nanoparticles can prevent oxidative damage to chloroplasts, maintain their structural integrity, and enhance photosynthetic efficiency (Wahab et al. 2023). Additionally, they can protect mitochondria from oxidative stress, ensuring efficient energy production and metabolic processes (Alam et al. 2021). Moreover, NPs can induce changes at the subcellular level, leading to improved stress tolerance. They can enhance the development and functionality of subcellular structures like peroxisomes and endoplasmic reticulum, which are involved in stress-related processes, including ROS detoxification and protein synthesis (Sharma et al. 2012). These improvements contribute to overall stress tolerance and plant performance (Cevik 2023). Through their interactions with plant cells, nanoparticles bring about ultra-structural changes that enhance the plant’s ability to cope with drought and salinity stress (Mariyam et al. 2024). These changes contribute to improved cellular integrity, water regulation, organelle protection, and subcellular functionality.
In conclusion, the exploration of nanoparticles in agriculture, as detailed in this review, represents a groundbreaking approach to enhancing the resilience of plants under stress. The unique attributes of nanoparticles, including their size, reactivity, and capacity for targeted delivery, present a spectrum of opportunities for stress mitigation. From improving nutrient uptake to triggering stress response mechanisms, nanoparticles show immense potential in fortifying plants against a range of environmental challenges such as drought, salinity, and metal toxicity. However, while the potential benefits are substantial, the environmental implications and potential health risks associated with nanoparticle use in agriculture must not be overlooked. The need for comprehensive understanding and careful regulation in the deployment of nanotechnology in agriculture is of utmost importance, to ensure the safety and sustainability of its applications. Further studies, particularly at the genomic, proteomic, and metabolomic levels, are required to unravel the detailed mechanisms of how different types of nanoparticles interact with plant systems under various environmental stress conditions strive to develop innovative, eco-friendly nanoparticles. Interdisciplinary approaches integrating nanotechnology, plant biology, and agricultural science are critical to realizing the potential of nanoparticles in promoting stress tolerance in plants. Thus, nanoparticles, when applied thoughtfully, can revolutionize agricultural practices, paving the way toward a future of increased plant resilience and food security.
Data availability
All data supporting the findings of this study are available within the paper and its supplementary materials published online.
References
Functional plant biology
Abasi F et al (2022) Biogenic silver nanoparticles as a stress alleviator in plants: a mechanistic overview. Molecules 27(11):3378
AbdalDayem A et al (2017) The role of Reactive Oxygen Species (ROS) in the biological activities of metallic nanoparticles. Int J Mol Sci 18(1):120
Abdel Latef AAH, Abu Alhmad MF, Abdelfattah KE (2017) The possible roles of priming with ZnO nanoparticles in mitigation of salinity stress in lupine (Lupinus termis) plants. J Plant Growth Regul 36:60–70
Abdel Latef AAH, Srivastava AK, El-sadek MSA, Kordrostami M, Tran L-SP (2018) Titanium dioxide nanoparticles improve growth and enhance tolerance of broad bean plants under saline soil conditions. Land Degrad Develop 29:1065–1073. https://doi.org/10.1002/ldr.2780
Abdel-Hakim SG et al (2023) Nanoparticulate fertilizers increase nutrient absorption efficiency and agro-physiological properties of lettuce plant. Agronomy 13(3):691
Agnihotri A et al (2018) Counteractive mechanism (s) of salicylic acid in response to lead toxicity in Brassica juncea (L.) Czern. Cv. Varuna. Planta 248(1):49–68
Alabdallah NM, Hasan MM (2021) Plant-based green synthesis of silver nanoparticles and its effective role in abiotic stress tolerance in crop plants. Saudi J Biol Sci 28(10):5631–5639
Alam P et al (2021) Silicon attenuates the negative effects of chromium stress in tomato plants by modifying antioxidant enzyme activities, ascorbate–glutathione cycle and glyoxalase system. 43(7):110
Alharbi K et al (2023) Zinc oxide nanoparticles and PGPR strengthen salinity tolerance and productivity of wheat irrigated with saline water in sodic-saline soil. Plant Soil 493(1–2):475–495
Al-Khayri JM et al (2023a) The role of nanoparticles in response of plants to abiotic stress at physiological, biochemical, and molecular levels. Plants (Basel) 12(2):292
Al-Khayri JM et al (2023b) The role of nanoparticles in response of plants to abiotic stress at physiological, biochemical, and molecular levels. 12(2):292
Al-Mamun MR et al (2021) Nanofertilizers towards sustainable agriculture and environment. Environ Technol Innov 23:101658
Amans D et al (2011) Synthesis of oxide nanoparticles by pulsed laser ablation in liquids containing a complexing molecule: impact on size distributions and prepared phases. J Phys Chem C 115(12):5131–5139
Aslani F et al (2014) Effects of engineered nanomaterials on plants growth: an overview. Sci World J 2014:641759
Azameti MK, Imoro A-WM (2023) Nanotechnology: a promising field in enhancing abiotic stress tolerance in plants. Crop Des 2(2):100037
Balusamy SR et al (2023) Advancing sustainable agriculture: a critical review of smart and eco-friendly nanomaterial applications. J Nanobiotechnol 21(1):372
Bano I et al (2021) Uses of selenium nanoparticles in the plant production. Agronomy 11(11):2229
Bayda S et al (2019) The history of nanoscience and nanotechnology: from chemical-physical applications to nanomedicine. Molecules 25(1):112
Berne BJ, Pecora R (2000) Dynamic light scattering: with applications to chemistry, biology, and physics. Courier Corporation
Bharath P, Gahir S, Raghavendra AS (2021) Abscisic acid-induced stomatal closure: an important component of plant defense against abiotic and biotic stress. Front Plant Sci 12:615114
Bokov D et al (2021) Nanomaterial by sol-gel method: synthesis and application. Adv Mater Sci Eng 2021:5102014
Bontempo P, De Masi L, Rigano D (2023) Functional properties of natural products and human health. Nutrients 15(13):2961
Bora KA et al (2022) Recent progress in bio-mediated synthesis and applications of engineered nanomaterials for sustainable agriculture. 13
Budhani S et al (2019) Phytotoxic effect of silver nanoparticles on seed germination and growth of terrestrial plants. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 37(4):330–355
Cevik S (2023) TiO2 nanoparticles alleviates the effects of drought stress in tomato seedlings. Bragantia 82
Chandrashekar HK et al (2023) Nanoparticle-mediated amelioration of drought stress in plants: a systematic review. 3 Biotech 13(10):336
Chaturvedi PK, Seth CS, Misra V (2006) Sorption kinetics and leachability of heavy metal from the contaminated soil amended with immobilizing agent (humus soil and hydroxyapatite). Chemosphere 64(7):1109–1114
Chaud M et al (2021) Nanopesticides in agriculture: benefits and challenge in agricultural productivity, toxicological risks to human health and environment. Toxics 9(6)
Chausali N, Saxena J, Prasad R (2021) Nanobiochar and biochar based nanocomposites: advances and applications. J Agric Food Res 5:100191
Chen J et al (2015) Nitric oxide ameliorates zinc oxide nanoparticles-induced phytotoxicity in rice seedlings. 297:173–182
Choudhary R et al (2024) Comprehensive journey from past to present to future about seed priming with hydrogen peroxide and hydrogen sulfide concerning drought, temperature, UV and ozone stresses- a review. Plant Soil
Cominelli E, Galbiati M, Tonelli C (2010) Transcription factors controlling stomatal movements and drought tolerance. Transcription 1(1):41–45
Dilnawaz F, Kalaji MH, Misra AN (2023) Nanotechnology in improving photosynthesis under adverse climatic conditions: cell to canopy action. Plant Nano Biol 4:100035
Dreaden EC et al (2012) The golden age: gold nanoparticles for biomedicine. Chem Soc Rev 41(7):2740–2779
Elemike EE et al (2019) The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl Sci 9(3):499
El-Saadony MT et al (2022) Role of nanoparticles in enhancing crop tolerance to abiotic stress: a comprehensive review. 13
Etesami H, Fatemi H, Rizwan M (2021) Interactions of nanoparticles and salinity stress at physiological, biochemical and molecular levels in plants: a review. Ecotoxicol Environ Saf 225:112769
Faizan M et al (2021) Zinc oxide nanoparticles (ZnO-NPs) induce salt tolerance by improving the antioxidant system and photosynthetic machinery in tomato. Plant Physiol Biochem 161:122–130
Fedlheim DL, Foss CA (2001) Metal nanoparticles: synthesis, characterization, and applications. CRC Press
Foyer CH (2018) Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ Exp Bot 154:134–142
Geremew A et al (2023) Effect of zinc oxide nanoparticles synthesized from Carya illinoinensis leaf extract on growth and antioxidant properties of mustard (Brassica juncea). 14
Goldstein JI et al (2003) Special topics in scanning electron microscopy. In: Goldstein JI, Newbury DE, Echlin P, Joy DC, Lyman CE, Lifshin E, Sawyer L, Michael JR (eds) Scanning electron microscopy and X-ray microanalysis, 3rd edn. Springer, US, Boston, pp 195–270
Gomaa MA et al (2021) Increase maize productivity and water use efficiency through application of potassium silicate under water stress. 11(1):1–8
Gujrati M et al (2014) Multifunctional cationic lipid-based nanoparticles facilitate endosomal escape and reduction-triggered cytosolic siRNA release. Mol Pharm 11(8):2734–2744
Gupta A et al (2023) Nanotechnology applications in sustainable agriculture: An emerging eco-friendly approach. Plant Nano Biol 4:100033
Hao S et al (2021) A review on plant responses to salt stress and their mechanisms of salt resistance. Horticulturae 7(6):132
Hayat F et al (2023) Nanoparticles and their potential role in plant adaptation to abiotic stress in horticultural crops: a review. Sci Hortic 321:112285
He X, Deng H, Hwang H-M (2019) The current application of nanotechnology in food and agriculture. J Food Drug Anal 27(1):1–21
Iavicoli I et al (2017) Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicol Appl Pharmacol 329:96–111
Irfan M et al (2023) Silicon nutrition in plants under water-deficit conditions: overview and prospects. Water 15(4):739
Joudeh N, Linke D (2022) Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J Nanobiotechnol 20(1):262
Junedi MA, Mukhopadhyay R, Sri Manjari K (2023) Alleviating salinity stress in crop plants using new engineered nanoparticles (ENPs). Plant Stress 9:100184
Karumannil S et al (2023) Impact of exogenous melatonin application on photosynthetic machinery under abiotic stress conditions. Plants 12(16):2948
Kataria S et al (2019) Chapter 6 - role of nanoparticles on photosynthesis: avenues and applications. In: Tripathi DK, Ahmad P, Sharma S, Chauhan DK, Dubey NK (eds) Nanomaterials in plants, algae and microorganisms. Academic Press, pp 103–127
Khalid MF et al (2022) Nanoparticles: the plant saviour under abiotic stresses. Nanomaterials 12(21):3915
Khan I, Saeed K, Khan I (2019) Nanoparticles: Properties, applications and toxicities. Arab J Chem 12(7):908–931
Khan I et al (2020) Amelioration of salt induced toxicity in pearl millet by seed priming with silver nanoparticles (AgNPs): the oxidative damage, antioxidant enzymes and ions uptake are major determinants of salt tolerant capacity. Plant Physiol Biochem 156:221–232
Khan I et al (2022) Nanoparticle’s uptake and translocation mechanisms in plants via seed priming, foliar treatment, and root exposure: a review. Environ Sci Pollut Res 29(60):89823–89833
Khanna K et al (2021) Enthralling the impact of engineered nanoparticles on soil microbiome: A concentric approach towards environmental risks and cogitation. Ecotoxicol Environ Saf 222:112459
Kim D-Y et al (2024a) Bioinspired silver nanoparticle-based nanocomposites for effective control of plant pathogens: A review. Sci Total Environ 908:168318
Kim D-Y et al (2024b) Alginic acid-functionalized silver nanoparticles: A rapid monitoring tool for detecting the technology-critical element tellurium. J Hazard Mater 465:133161
Kookana RS et al (2014) Nanopesticides: guiding principles for regulatory evaluation of environmental risks. J Agric Food Chem 62(19):4227–4240
Kumar D et al (2023) Titanium dioxide nanoparticles potentially regulate the mechanism(s) for photosynthetic attributes, genotoxicity, antioxidants defense machinery, and phytochelatins synthesis in relation to hexavalent chromium toxicity in Helianthus annuus L. J Hazard Mater 454:131418
Kumar D et al (2024) Review on interactions between nanomaterials and phytohormones: novel perspectives and opportunities for mitigating environmental challenges. Plant Sci 340:111964
Kumari S et al (2022) Bio-synthesized nanoparticles in developing plant abiotic stress resilience: a new boon for sustainable approach. Int J Mol Sci 23(8):4452
Kumari R et al (2023) Regulation and safety measures for nanotechnology-based agri-products. 5
Kuppusamy P et al (2016) Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications - an updated report. Saudi Pharm J 24(4):473–484
Li D et al (2022a) Wearable crop sensor based on nano-graphene oxide for noninvasive real-time monitoring of plant water. Membranes (Basel) 12(4):358
Li S et al (2022b) The interaction of ABA and ROS in plant growth and stress resistances. Front Plant Sci 13:1050132
Liang Y et al (2022) Antagonistic skin toxicity of co-exposure to physical sunscreen ingredients zinc oxide and titanium dioxide nanoparticles. Nanomaterials 12(16):2769
Lin D et al (2016) An overview of plant phenolic compounds and their importance in human nutrition and management of type 2 diabetes. Molecules 21(10):1374
Liu R, Lal R (2015) Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci Total Environ 514:131–139
Long CM, Nascarella MA, Valberg PA (2013) Carbon black vs. black carbon and other airborne materials containing elemental carbon: physical and chemical distinctions. Environ Pollut 181:271–286
Lu K-Q et al (2016) Multifarious roles of carbon quantum dots in heterogeneous photocatalysis. J Energy Chem 25(6):927–935
Maharramov AM et al (2019) The engineered nanoparticles in food chain: potential toxicity and effects. SN Appl Sci 1(11):1362
Mahmoud LM et al (2020) Silicon nanoparticles mitigate oxidative stress of in vitro-derived banana (Musa acuminata ‘Grand Nain’) under simulated water deficit or salinity stress. S Afr J Bot 132:155–163
Manzoor N et al (2023) Comparative efficacy of silicon and iron oxide nanoparticles towards improving the plant growth and mitigating arsenic toxicity in wheat (Triticum aestivum L.). Ecotoxicol Environ Saf 264:115382
Mariyam S et al (2023) Review on nitric oxide at the forefront of rapid systemic signaling in mitigation of salinity stress in plants: Crosstalk with calcium and hydrogen peroxide. Plant Sci 336:111835
Mariyam S et al (2024) Nanotechnology, a frontier in agricultural science, a novel approach in abiotic stress management and convergence with new age medicine-a review. Sci Total Environ 912:169097
Mauter MS, Elimelech M (2008) Environmental applications of carbon-based nanomaterials. Environ Sci Technol 42(16):5843–5859
Mittal D et al (2020) Nanoparticle-based sustainable agriculture and food science: recent advances and future outlook. 2
Mochalin VN et al (2012) The properties and applications of nanodiamonds. Nat Nanotechnol 7(1):11–23
Mohammadi H et al (2023) Foliar applied titanium dioxide nanoparticles (TiO2 NPs) modulate growth, physiological and phytochemical traits in Melissa officinalis L. under various light intensities. Ind Crops Prod 197:116642
Moreno-Vega A-I et al (2012) Polymeric and ceramic nanoparticles in biomedical applications. J Nanotechnol 2012:936041
Mukarram M et al (2022) Silicon nanoparticles in higher plants: Uptake, action, stress tolerance, and crosstalk with phytohormones, antioxidants, and other signalling molecules. Environ Pollut 310:119855
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681
Nadarajah KK (2020) ROS homeostasis in abiotic stress tolerance in plants. Int J Mol Sci 21(15):5208
Nair PM, Chung IM (2014) Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignificaion, and molecular level changes. Environ Sci Pollut Res Int 21(22):12709–12722
Nascimento MA et al (2018) Synthesis of polymetallic nanoparticles from spent lithium-ion batteries and application in the removal of reactive blue 4 dye. J Clean Prod 202:264–272
Nayeri S et al (2023) Ag/ZnO core–shell NPs boost photosynthesis and growth rate in wheat seedlings under simulated full sun spectrum. Sci Rep 13(1):14385
Ng KK, Zheng G (2015) Molecular Interactions in Organic Nanoparticles for Phototheranostic Applications. Chem Rev 115(19):11012–11042
Noga M et al (2023) Toxicological Aspects, Safety Assessment, and Green Toxicology of Silver Nanoparticles (AgNPs)-critical review: state of the art. Int J Mol Sci 24(6):5133
Nongbet A et al (2022) Nanofertilizers: a smart and sustainable attribute to modern agriculture. Plants 11(19):2587
Okeke ES et al (2023) Silicon-based nanoparticles for mitigating the effect of potentially toxic elements and plant stress in agroecosystems: A sustainable pathway towards food security. Sci Total Environ 898:165446
Padmanaban S et al (2023) Rising influence of nanotechnology in addressing oxidative stress-related liver disorders. Antioxidants (Basel) 12(7):1405
Pan K, Zhong Q (2016) Organic nanoparticles in foods: fabrication, characterization, and utilization. Annu Rev Food Sci Technol 7:245–266
Patel M (2022) Green synthesis of nanoparticles: a solution to environmental pollution. In: Baskar C, Ramakrishna S, Baskar S, Sharma R, Chinnappan A, Sehrawat R (eds) Handbook of solid waste management: sustainability through circular economy. Springer Nature Singapore, Singapore, pp 1965–1993
Patra S et al (2022) Prospects of hydrogels in agriculture for enhancing crop and water productivity under water deficit condition. Int J Polym Sci 2022:1–15
Pecharsky VK, Zavalij PY (n.d.) Fundamentals of powder diffraction and structural characterization of materials [electronic resource]
Prajapati P et al (2023) Nitric oxide mediated regulation of ascorbate-glutathione pathway alleviates mitotic aberrations and DNA damage in Allium cepa L. under salinity stress. Int J Phytoremediation 25(4):403–414
Prasad R, Bhattacharyya A, Nguyen QD (2017) Nanotechnology in Sustainable Agriculture: Recent Developments, Challenges, and Perspectives. Front Microbiol 8:1014
Qi M, Liu Y, Li T (2013) Nano-TiO 2 improve the photosynthesis of tomato leaves under mild heat stress. Biol Trace Elem Res 156:323–328
Rai PK, Song H, Kim K-H (2023) Nanoparticles modulate heavy-metal and arsenic stress in food crops: Hormesis for food security/safety and public health. Sci Total Environ 902:166064
Raliya R, Biswas P, Tarafdar JC (2015) TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L). Biotechnol Rep 5:22–26
Ramadan T et al (2022) The combined effect of water deficit stress and TiO2 nanoparticles on cell membrane and antioxidant enzymes in Helianthus annuus L. Physiol Mol Biol Plants 28(2):391–409
Rasheed A et al (2022a) The role of nanoparticles in plant biochemical, physiological, and molecular responses under drought stress: a review. Front Plant Sci 13:976179
Rasheed A et al (2022b) The role of nanoparticles in plant biochemical, physiological, and molecular responses under drought stress: A review. Front Plant Sci 13
Rasheed A et al (2022c) The role of nanoparticles in plant biochemical, physiological, and molecular responses under drought stress: A review. 13
Ray PC, Yu H, Fu PP (2009) Toxicity and environmental risks of nanomaterials: challenges and future needs. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 27(1):1–35
Raza A et al (2023) Nano-enabled stress-smart agriculture: Can nanotechnology deliver drought and salinity-smart crops? J Sustain Agric Environ 2(3):189–214
Rehman A et al (2023) Green synthesized zinc oxide nanoparticles confer drought tolerance in melon (Cucumis melo L.). Environ Exp Bot 212:105384
Rehman A et al (2024) Exploring the nano-wonders: unveiling the role of Nanoparticles in enhancing salinity and drought tolerance in plants. 14
Rico CM et al (2011) Interaction of nanoparticles with edible plants and their possible implications in the food chain. J Agric Food Chem 59(8):3485–3498
Rizwan M et al (2017) Effect of metal and metal oxide nanoparticles on growth and physiology of globally important food crops: a critical review. J Hazard Mater 322:2–16
Rizwan M et al (2019) Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 214:269–277
Saba N et al (2023) Improvement of abiotic stress tolerance in plants with the application of nanoparticles. In: Manuel O, Anabela F-S (eds) Abiotic stress in plants. Rijeka, IntechOpen, p 9
Saba N et al (2023) Improvement of abiotic stress tolerance in plants with the application of nanoparticles. In: Manuel TTO, Afonso AFSA (eds) Abiotic stress in plants - adaptations to climate change. Rijeka, IntechOpen, p 1
Samanta S, Seth CS, Roychoudhury A (2024) The molecular paradigm of reactive oxygen species (ROS) and reactive nitrogen species (RNS) with different phytohormone signaling pathways during drought stress in plants. Plant Physiol Biochem 206:108259
Saritha GN, Gari TA, Kumar A (2022) Nanotechnology - Big impact: How nanotechnology is changing the future of agriculture? J Agric Food Res 10:100457
Sasaki T, Shimizu Y, Koshizaki N (2006) Preparation of metal oxide-based nanomaterials using nanosecond pulsed laser ablation in liquids. J Photochem Photobiol, A 182(3):335–341
Seleiman MF et al (2021) Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants (Basel) 10(2)
Seleiman MF et al (2023) Foliar applications of ZnO and SiO2 nanoparticles mitigate water deficit and enhance potato yield and quality traits. 13(2):466
Selvakesavan RK et al (2023) Impact of nanomaterials on plant secondary metabolism. In: Al-Khayri JM, Alnaddaf LM, Jain SM (eds) Nanomaterial interactions with plant cellular mechanisms and macromolecules and agricultural implications. Springer International Publishing, Cham, pp 133–170
Shah T et al (2021) Seed priming with titanium dioxide nanoparticles enhances seed vigor, leaf water status, and antioxidant enzyme activities in maize (Zea mays L.) under salinity stress. J King Saud Univ - Sci 33(1):101207
Shang Y et al (2019) Applications of nanotechnology in plant growth and crop protection: a review. Molecules 24(14)
Sharma P et al (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot 2012:217037
Sharma A et al (2019) Phytohormones regulate accumulation of osmolytes under abiotic stress. Biomolecules 9(7):285
Shelar A et al (2023) Recent advances in nano-enabled seed treatment strategies for sustainable agriculture: challenges, risk assessment, and future perspectives. Nano-Micro Lett 15(1):54
Sherin G, Raj Aswathi KP, Puthur JT (2022) Photosynthetic functions in plants subjected to stresses are positively influenced by priming. Plant Stress 4:100079
Sheteiwy MS et al (2021) Zinc oxide nanoparticles: potential effects on soil properties, crop production, food processing, and food quality. Environ Sci Pollut Res 28(28):36942–36966
Singh J, Lee B-K (2016) Influence of nano-TiO2 particles on the bioaccumulation of Cd in soybean plants (Glycine max): A possible mechanism for the removal of Cd from the contaminated soil. J Environ Manag 170:88–96
Singh J et al (2018) ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol 16(1):84
Singh A et al (2022) Zinc oxide nanoparticles improve salt tolerance in rice seedlings by improving physiological and biochemical indices. 12(7):1014
Spanò C et al (2020) Effect of zinc priming on salt response of wheat seedlings: relieving or worsening? Plants (Basel) 9(11)
Stuart B (2021) Infrared spectroscopy. Analytical Techniques in Forensic Science. pp 145–160
Sun S et al (2000) Monodisperse FePt nanoparticles and ferromagnetic FePt nanocrystal superlattices. 287(5460):1989–1992
Tan W et al (2017) Surface coating changes the physiological and biochemical impacts of nano-TiO2 in basil (Ocimum basilicum) plants. Environ Pollut 222:64–72
Tariq M et al (2022) Biological synthesis of silver nanoparticles and prospects in plant disease management. Molecules 27(15):4754
Thabet SG, Alqudah AM (2023) New genetic insights into improving barley cope with salt stress via regulating mineral accumulation, cellular ion homeostasis, and membrane trafficking. Environ Exp Bot 208:105252
Thabet SG et al (2021a) Genetic factors controlling nTiO(2) nanoparticles stress tolerance in barley (Hordeum vulgare) during seed germination and seedling development. Funct Plant Biol 48(12):1288–1301
Thabet SG, Alomari DZ, Alqudah AM (2021b) Exploring natural diversity reveals alleles to enhance antioxidant system in barley under salt stress. Plant Physiol Biochem 166:789–798
Thomas SC et al (2015) Ceramic Nanoparticles: Fabrication Methods and Applications in Drug Delivery. Curr Pharm Des 21(42):6165–6188
Toshima N, Yonezawa T (1998) Bimetallic nanoparticles—novel materials for chemical and physical applications. New J Chem 22(11):1179–1201
Tripathi DK et al (2017) Silicon nanoparticles more effectively alleviated UV-B stress than silicon in wheat (Triticum aestivum) seedlings. Plant Physiol Biochem 110:70–81
Trono D, Pecchioni N (2022) Candidate genes associated with abiotic stress response in plants as tools to engineer tolerance to drought, salinity and extreme temperatures in wheat: an overview. Plants (Basel) 11(23):3358
Upadhayay VK et al (2023) Synergistic impact of nanomaterials and plant probiotics in agriculture: A tale of two-way strategy for long-term sustainability. Front Microbiol 14:1133968
Vannini C et al (2014) Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J Plant Physiol 171(13):1142–1148
Vijayakumar MD et al (2022) Evolution and recent scenario of nanotechnology in agriculture and food industries. J Nanomater 2022:1280411
Vittori Antisari L et al (2015) Uptake and translocation of metals and nutrients in tomato grown in soil polluted with metal oxide (CeO 2, Fe 3 O 4, SnO 2, TiO 2) or metallic (Ag, Co, Ni) engineered nanoparticles. 22:1841–1853
Wahab A et al (2023) Current knowledge, research progress, and future prospects of phyto-synthesized nanoparticles interactions with food crops under induced drought. Stress 15(20):14792
Wang L, Hu C, Shao L (2017) The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomedicine 12:1227–1249
Wang X et al (2023) Nanoparticles in plants: uptake, transport and physiological activity in leaf and root. Materials (Basel) 16(8):3097
Yadav A, Yadav K, Abd-Elsalam KA (2023a) Nanofertilizers: types, delivery and advantages in agricultural sustainability. 2(2):296-336
Yadav A et al (2023b) Emerging frontiers in nanotechnology for precision agriculture: advancements, hurdles and prospects. 2(2):220-256
Yan A, Chen Z (2019) Impacts of silver nanoparticles on plants: a focus on the phytotoxicity and underlying mechanism. Int J Mol Sci 20(5):1003
Yasmin H et al (2021) Combined application of zinc oxide nanoparticles and biofertilizer to induce salt resistance in safflower by regulating ion homeostasis and antioxidant defence responses. Ecotoxicol Environm Saf 218:112262
Yin L et al (2012) Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE 10(7):e47674
Zanella D et al (2017) Iron oxide nanoparticles can cross plasma membranes. Sci Rep 7(1):11413
Zhao B et al (2022) Imaging tools for plant nanobiotechnology. Front Genome Ed 4:1029944
Zhu F et al (2022) Microplastics altered soil microbiome and nitrogen cycling: The role of phthalate plasticizer. J Hazard Mater 427:127944
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yongchao Liang.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlight
The application of nanoparticles (NPs) in plant biology, particularly in addressing biotic and abiotic stress conditions in different plant species, has become an intriguing field of research. The small size of NPs allows for increased interaction with biological molecules, presenting opportunities for targeted delivery of nutrients, disease management, and stress mitigation.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thabet, S.G., Alqudah, A.M. Unraveling the role of nanoparticles in improving plant resilience under environmental stress condition. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06581-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06581-2