Abstract
Background and aims
This field-base study evaluates the long-term effectiveness of in-situ remediation measures applied to soils residually polluted by potentially toxic elements (PTEs) in an area affected by a mining spill in SW Spain.
Methods
To evaluate the remediation treatments success, their influence on key soil properties and on the development of spontaneous vegetation in the treated soils was investigated. The treatments were based on human derived by-products valorization, and consisted of: biopiles, marble sludge and gypsum mining spoil addition, and their combination with an organic amendment (vermicompost).
Results
Amendments application improved the soil properties and reduced PTEs availability. As a result, an enhancement in spontaneous development of vegetation cover and diversity of plant species in the treated soils was followed. Spergularia rubra and Lamarckia aurea, two primary plant species growing in the studied area and that exhibit strong association to soils with the highest levels of pollution, showed high Pb and As accumulation in shoots ande in roots. Exceptionally, accumulation of these pollutants occurred in L. aurea roots, which can explain its high presence in soils with more limited vegetation development and in which no additional plant species can thrive.
Conclusions
The occurrence of S. rubra and L. aurea in the amended soils may be indicative of improved soil conditions and reduced toxicity induced by the remediation measures implemented. They may also be considered key species in the area since their presence can promote the recolonization of the degraded soils by species less tolerant to their residual pollution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Soil, as a fundamental element of ecosystems, plays a crucial role in providing essential environmental functions and ecosystem services. Among these, soil productivity is one of the most essential services for the human well-being and its development (Kabata-Pendias 2010; Adhikari and Hartemink 2016). Nevertheless, soil faces several soil degradation processes that may threaten its quality, both natural and human-induced. Of the anthropogenic causes, soil pollution by potentially toxic elements (PTEs) represent one of the major threats and concerns worldwide (FAO and ITPS 2015), since it poses a risk to ecological systems, human health, and food production. Among all chemical pollutants, PTEs, including heavy metals and metalloids, can persist tightly bound in soil (Pilon-Smits 2005; Igalavithana et al. 2022). This can lead to cumulative impacts in organisms, ultimately making them a significant contributor to environmental pollution with implicit adverse effects to human health (Muyessar and Linsheng 2016). Although certain PTEs can act as essential elements and are involved in biological functions, such as Cu and Zn, they can turn potentially toxic when specific thresholds are exceeded (Gall et al. 2015). Other elements such as As and Pb are among the most hazardous PTEs, as very low concentrations of them can cause toxicity (Rahman and Singh 2019), causing serious problems of contamination in the food chain, with the consequent health risk (Simon 2014). Therefore, in order to preserve soil functions, degraded soil ecosystems polluted by PTEs would require assisted natural remediation, ANR (Adriano et al. 2004; Raklami et al. 2021).
With the aim of reducing the PTEs harmful effects in polluted soils, a wide range of techniques for soil remediation, either in-situ or ex-situ, and involving the use of both organic and inorganic amendments, have been developed and tested to assist natural remediation processes (Adriano et al. 2004; Park et al. 2011; Liu et al. 2018). The addition of amendments to the soil is considered as an economical and environmentally efficient solution that addresses pollutant toxicity and enhances critical biogeochemical mechanisms (Rodríguez-Jordá et al. 2012; Nirola et al. 2016). Moreover, the revalorization of mining and agro-food industry wastes through their application as amendments in degraded soils aligns with zero-waste strategies (Greyson 2007; Pietzsch et al. 2017). Numerous case studies corroborate the viability of organic and/or inorganic soil amendments under field conditions (Fernández-Caliani and Barba-Brioso 2010; González et al. 2012). More precisely, liming or calcium and organic matter-rich amendments are among the most effective ones, through the correction of soil acidic pH, the enhancement of soil physicochemical properties, the increase of nutrient availability and the immobilization of certain PTEs (Bernal et al. 2007; Pérez-de-Mora et al. 2007), which prevent from the potential spread of pollutants into the ecosystem. Consequently, a promotion in re-colonization and re-establishment in barren polluted soils by the vegetation from surrounding areas in the remediated soils may be achieved in turn (Fernández-Caliani and Barba-Brioso 2010; Xiong et al. 2015) through the enhancement of plant germination and growth (Clemente et al. 2015; Madejón et al. 2018; Sierra-Aragón et al. 2019).
Industrial activities, such as mining, represent one of the most significant potential sources of soil pollution by PTEs (Dermont et al. 2008; Liu et al. 2018), and several remediation treatments have been implemented in mining polluted areas. One of the greatest metal mining accidents worldwide took place in 1998 in Spain, after the collapse of the tailings dam of the Aznalcóllar pyrite mine, which resulted in the spill of acidic waters and tailings containing high concentrations of PTEs (Grimalt et al. 1999; Simón et al. 1999; Sanz-Ramos et al. 2022). Following, extensive cleanup and rehabilitation measures were implemented in the affected area to guarantee the safety of the local inhabitants and promote the full recovery of the area in the long term. However, several decades after the accident, numerous patches of residual polluted soils where vegetation cannot even germinate still remain in the area, and represent an environmental risk for their high PTEs concentrations (García-Carmona et al. 2017). Since the residual polluted patches are deeply integrated into a large recovered area (the Protected Landscape of Guadiamar Green Corridor), the mixture of adjacent recovered soil with the remaining polluted soil represents a plausible, economic, and minimally invasive technique. This treatment has been tested in previous experiments in the area with successful results directly tied to the improvement of soil properties and reduced PTEs solubility (García-Carmona et al 2017; Lorente-Casalini et al. 2021).
When evaluating the restoration processes of degraded or polluted areas, the presence of key components of ecosystems such as vegetation plays a crucial role as indicators of the extent of soil pollution. Trace elements, and particularly heavy metals, can be accumulated by plants, which are able to adapt to varying levels of environmental pollution (Kabata-Pendias 2010). Furthermore, passive restoration, which involves the natural recolonization of a polluted area by native vegetation and that ocurres in parallel to the conditions improvement, is crucial when assessing the effectiveness of remediation measures (Álvarez-Rogel et al. 2021), as these native plants may act as “nurse plants”, promoting the growth of species with lower tolerance to stress conditions (Navarro-Cano et al. 2018). Additionally, the development of vegetation cover is relevant when considering the effectiveness of a specific treatment applied to a polluted soil, since it provides an extra physical protection and may reduce possible re-movement of pollutant particles and migration to groundwater (Pérez-de-Mora et al. 2006). However PTEs toxicity in plants can limit processes such as seed germination, root development, and growth, or disrupt the uptake of essential nutrients leading to plant death (Kabata-Pendias 2010). Nonetheless, a wide variety of strategies, both physiological and behavioral, allow numerous plant species to tolerate remarkably high PTEs concentrations (Baker et al. 2010; Viehweger 2014), such as metallophytes species inhabiting metalliferous deposits (Baker et al. 2010). Also, certain plant species not exclusive to metal-rich environments, known as pseudometallophytes (Baker et al. 2010), also exibit exceptional capacity to adapt to unfavourable soil conditions caused by pollution. Under moderate levels of soil metal toxicity, they can show higher biomass production and have competitive advantages over other species (Poschenrieder et al. 2001). Consequently, these species may contribute to improve the physicochemical and biological conditions of the polluted soils by handling the PTEs concentrations, enhancing nutrient availability, and increasing soil organic carbon (Arienzo et al. 2004; Bolan et al. 2011).
The aim of this study is to evaluate the long-term effects of in-situ remediation measures, based on the addition of inorganic and organic amendments (six different treatments), which were applied to soils residually polluted by PTEs. The influence of the treatments will be determined by analyzing: i) the changes induced in the main soil properties; ii) the mobility and bioavailability of PTEs after remediation and ageing; iii) the natural settling of PTEs tolerant plants, as an indicator of the success of the remediation actions used; and iv) the bioaccumulation and translocation factors of PTEs in these tolerant plant species, for a better understanding of the mechanisms that allow them to survive to the great concentrations present in the studied soils.
Material and methods
Study site and experimental design
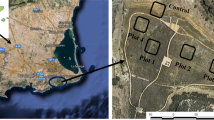
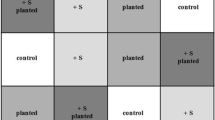
The study site was located in the area nearby the tailings dam of the Aznalcóllar mine (Seville, SW Spain), which was most severely affected by the Aznalcóllar mining spill. This area is characterized by the presence of heterogeneously distributed bare soil patches of varying sizes, where high levels of pollution are still detected (Fig. 1a and b). For this study, three of these unvegetated residually polluted soil patches were selected (Fig. 1c). Within each of these patches, an experimental plot of 24 m2 was set up, and divided into six subplots of 4 m2 with a different treatment applied in each of them (Fig. 1d). The six treatments selected were the following: 1. BS: Biopile (50% w/w mixture of polluted soil -PS- with adjacent recovered soil -RS-); 2. BVS: Biopile + vermicompost; 3. GS: Gypsum mining spoil (5 kg m−2); 4. GVS: Gypsum mining spoil + vermicompost; 5. MS: Marble sludge (5 kg m−2); 6. MVS: Marble sludge + vermicompost. The doses applied of every amendment was 5 kg m−2 for each subplot. The selected amendments were chosen for being calcium and organic matter-rich amendments, and in the case of the inorganic amendments, for representing low-cost waste materials generated in very large amounts that need to be sustainably managed (Rayed et al. 2019; García-Robles et al. 2022). The doses of gypsum mining spoil and marble sludge were selected according to the doses previously applied in the area for calcium-rich amendments (Madejón et al. 2018), and the ratio for the BS treatment was successfully tested in a previous ex-situ experiment (García-Carmona et al. 2017). In all cases, the dose of vermicompost was 5 kg m−2 (equivalent to 50 t ha−1) because the organic amendments had been previously applied in the area at a rate of 15–25 t ha−1 (Cabrera et al. 2005, 2008) and resulted insufficient to promote vegetation growth. The characterization of the amendments (biopile, vermicompost, marble sludge and gypsum mining spoil) is shown in Table S1.
Soil and vegetation sampling
After a period of eighteen months following the implementation of the treatments, coinciding with the second spring, surface soil composite samples (0 – 10 cm) for each treatment were collected, and the key soil properties of them were determined. In addition, composite samples were also taken from untreated polluted soil (PS) within the unvegetated soil patches, from reclaimed soil adjacent to them (RS), and from soils unaffected by the spill near the studied area (US), which were considered reference soils for the different degrees of pollution. Moreover, vegetation cover and plant species richness present in every reference soil (in a representative 4 m2 surface) and treatment applied were measured to monitor the vegetation development in the plots. This was done using a 0.25 m2 quadrat divided into 100 cells, which was placed on the surface of each of the studied soil, and all the cells with presence of a herbaceous species were counted, both for total vegetation cover and for the specific cover of each species individually. Also, richness, considered as the mean total number of different species encountered on the quadrat, was determined for each treatment. This procedure was performed in triplicate for each experimental plot and treatment. Moreover, to analyze the potentially toxic elements (PTEs) uptake by the main colonizing species in the plots, samples of Spergularia rubra (L.) J. Presl & C. Presl and Lamarckia aurea (L.) Moench were collected.
Analytical methods
Soil properties
Soil samples were air dried at room temperature and sieved (< 2 mm). The main soil properties and constituents including pH, electrical conductivity, calcium carbonate content, and organic carbon content were analyzed following standard methods (MAPA 1994). Soil pH was determined in a soil/water ratio 1:2.5 with a 914 pH/Conductometer Metrohm (Herisau, Switzerland); electrical conductivity (EC, dS/m) in a soil/water extract (1:5) using a Eutech CON700 conductivity-meter (Oakton Instruments, Vernon-Hills, IL, USA); calcium carbonate content (%CaCO3) was measured by manometric method according to Barahona (1984); and organic carbon (%OC) was quantified by acid-oxidation method according to Tyurin (1951).
Total concentration and selective extractions of PTEs in soils
Total concentration of the main PTEs (xx_T) present in the studied soils (Pb, As, Zn, Cu) were determined by X-ray fluorescence (XRF) with a NITON XL3t-980 GOLDD + instrument (Thermo Fisher Scientific, Tewksbury, MA, USA) (n = 3). The accuracy of the method was confirmed by analyzing a certified reference material (CRM052-050 RT-Corporation Limited, Salisbury, UK; n = 6). The average recoveries for the studied elements were 108.0 ± 9% of the CRM. Also, selective extractions of PTEs were performed in all treatments to assess the risk of mobility and the bioavailability of these elements in soils. In this sense, a soil:water extract of 1:5 was prepared to obtain the PTEs water-soluble fraction (xx_W) (Gomez-Eyles et al. 2011), since this extraction simulates the proportion of the elements that can lixiviate with rain water (Romero-Baena et al. 2021). Meanwhile, a extraction with 0.05 M EDTA (pH = 7) was followed to obtain the bioavailable fraction (xx_EDTA) for living organisms (Quevauviller et al. 1998). The PTEs concentrations for both extracted forms were measured by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) in a PerkinElmer NexION® 300D spectrometer (Waltham, MA, USA). The accuracy of the method for extracted forms was also confirmed by analyzing certified reference material SPS-SW1 Batch 133 (Spectrapure Standards, Manglerud, Norway; n = 6). The recovery of the reference material for the studied elements by ICP-MS determination was 99.2 ± 0.8%.
PTEs concentration in plants
Collected plant samples were divided into shoots and roots to analyze them separately, washed with distilled water, and dried (70 °C for 48 h). Afterwards, all plant material was finely ground and digested in a microwave XP1500Plus (Mars®) in HNO3:H2O2 (1:1) (Sah and Miller 1992), and the concentration of the main PTEs in each part of the plant was measured by ICP-MS (PerkinElmer NexION® 300D spectrometer). The bioavailable (EDTA-extractable) PTEs fraction in soil samples was selected to calculate the bioaccumulation factor (BAF) for both parts, to assess the capacity of plants to uptake PTEs from the soil and either transport them to the shoots or accumulate them in roots. BAF, therefore, was calculated as the ratio between the PTEs concentration measured in plant (mg kg−1 dry weight) and the EDTA-extractable PTEs concentration in soils (mg kg−1 dry soil) (Anning and Akoto 2018; García-Carmona et al. 2019), since this fraction represents PTEs bioavailable forms for plants (Kidd et al. 2007). Finally, the extent of elements migration from roots to shoots was estimated through the translocation factor (TF), calculated as the ratio between PTEs concentration in shoots to that in the roots of the plants (Zacchini et al. 2009; Boi et al. 2021). Plants with both factors > 1 for a given element are considered as accumulators and suitable for phytoextraction, while plants with both factors < 1 are considered as non-accumulators or pollutant-stabilizing, thus being suitable for phytostabilization (Buscaroli 2017).
Statistical analyses
Descriptive statistics of PTEs content in soils and plant material were calculated to check their normality, while homogeneity of variance was explored using Levene's test. The analysis of mean comparisons was performed by non-parametric Kruskal–Wallis test, in accordance to sample size (Theodorsson-Norheim 1986). Kruskal–Wallis post hoc test (p < 0.05) was used to determine significant differences. Principal component analysis (PCA) was carried out to identify the relations between soil properties, PTEs fractions, and treatments. Also, to analyze the relation between PTEs concentrations in plants and their total and bioavailable concentrations in soils, Spearman’s correlations were performed. All the statistical analyses were carried out with a confidence level of 95% using SPSS v.28.0 (SPSS Inc., Chicago, USA).
Results and discussion
Main soil properties related to pollution
The key soil properties of the different reference soils and under each of the treatments applied are shown in Table 1. Soils with high levels of pollution remaining (PS) were mainly characterized by a strongly acidic pH (3.5), high EC (> 3 dS/m), and low organic carbon content (< 1%) due mainly to the lack of vegetation cover. In contrast, both the recovered soil (RS) and the unpolluted soil (US) were characterized by a less acidic pH, especially in US (> 6), significantly lower EC (< 1 dS/m), and significantly higher organic carbon content than PS (> 2%). These results are consistent with the ones reported by Sierra-Aragón et al. (2019) in polluted soils within the same area. Regarding the treated soils, the pH in them was higher compared to PS and to RS in all cases, and even reached higher values under marble (MS, MVS) and gypsum (GS, GVS) treatments than those of US. However, EC did not experience a strong decline in the amended soils, and values remained significantly higher than those of RS and US. The organic carbon content in amended soils did not reach that of the RS and US either, although among treatments it was higher in soils where vermicompost was added, for the direct effect of this organic amendment (Adak et al. 2014; Shen et al. 2022). Overall, US and RS were more strongly associated to a higher content of OC while PS was inversely correlated to OC content and pH, and, as for treatments, the strongest correlation was that of MS and MVS with a more neutral pH, as well as with the highest content in calcium carbonate (Fig. S1).
Total, water-soluble and EDTA-extractable PTEs concentrations
When assessing the effectiveness of remediation measures and the risk to the ecosystem of the polluted soils treated, determining PTEs fractions is critical. Even after soil remediation and recolonization by vegetation, acid releases may occur, so that enough liming or other amendment material should be applied to buffer against these situations, which can represent a threat to future scenarios in the remediated soils (Wong et al. 1998). The total concentrations measured in the unpolluted soils (US) can be considered as the background levels for the potentially toxic elements (PTEs) present in the soils of the study area (Table 2). The mean total concentrations for Pb, As, Zn and Cu in US were similar to the background levels recorded in the same area both shortly after the accident (Simón et al. 1999) and by more recent studies (García-Robles et al. 2022), although Pb showed slightly higher values in this occasion, which may be explained by the natural variability of this element found in the natural soil. Recovered soils (RS) showed total PTEs concentrations approximately fivefold higher than those of US for Pb, Zn and Cu, and almost 15-fold higher for As. In addition, RS showed the highest concentrations for Zn and Cu, while for As and Pb the highest concentrations were reached in PS, 10- and 23-times above background levels, respectively. When comparing the levels measured for these elements with the regulatory thresholds established by the Regional Government of Andalusia (BOJA 2015) [10,000 mg kg−1 for Zn, 595 mg kg−1 for Cu 36 mg kg−1 for As, and 275 mg kg−1 for Pb] the concentrations of As and Pb both in PS and RS widely exceeded these regulatory limits.
In relation to treatments, the dilution effect of the amendments over total PTEs concentrations was relevant for some elements, especially in biopile soils (BS and BVS). In this sense, total Pb and As concentrations were strongly reduced in BS and BVS compared to PS, showing concentrations similar to those in RS. Meanwhile, Zn levels in these treatments were intermediate compared to PS, while for Cu no changes were observed after the application of the treatments, indicating that the dilution effect for this element was not a determining factor. The rest of the amended soil treatments showed higher As and Pb total concentrations compared to RS, although still significantly lower than those in PS. Thus, PS was strongly related to high total concentrations of As and Pb, while RS did so for Zn and Cu total concentrations. This may be related to the association of Cu and Zn with the OC content, related to a specific binding by adsorption and complexation to soil organic matter (McLaren et al. 1981; Balasoiu et al. 2001; Yin et al. 2002), which led the RS, where the highest OC was found, to retain higher total concentrations of these elements. Moreover, the low total concentrations of Cu and Zn found in PS may be related to the increase in mobility of these elements with decreased soil pH (Wang et al. 2006; Zeng et al. 2011). Therefore, the acidic pH in PS could have led to a strong leaching of these elements at depths below the top 10 cm sampled.
With the aim to accurately evaluate PTEs toxicity in the soil, particular emphasis should be given to their solubility and bioavailability, which represent the fractions that are available for uptake by living organisms and that can spread within the soil and through the landscape, thus posing a potential toxicity risk to terrestrial ecosystems (Alibrahim and Williams 2016; Bagherifam et al. 2019). In this regard, water-soluble fraction of pollutants can be readily absorbed by plants and incorporated into the food chain by bioaccumulation, representing actual bioavailability and short-term metal dynamics. Moreover, Ghosh et al. (2004) found that water-soluble form of As was more toxic compared to total As and inflicted greater inhibitory effect on various microbiological parameters in polluted soils compared to other bioavailable forms. Consequently, water-soluble As may be indicative of As availability to microbial populations (Fernández et al. 2005). Concerning this fraction, the highest water-soluble concentrations of As and Pb were recorded in RS, while for Zn and Cu they were recorded in PS (Table 3), in contrast to what was observed for total concentrations of these PTEs. The high solubility of Zn and Cu under acidic conditions led to the high water-soluble concentrations found in PS. Meanwhile, higher As and Pb solubility in RS can be related to the high organic matter content in this soil, as well as to its specific composition, since these elements are usually related to the labile dissolved organic carbon (DOC) pool (Egli et al. 2010; Gangloff et al. 2014; Li et al. 2018). Thus, by different competing effects with these elements, organic matter in soil can lead to an increase in their availability by maintaining them in more soluble and labile forms (Sauvé et al. 2000; Sierra-Aragón et al. 2019).
Regarding treatments, Pb solubility strongly decreased in all treated soils, especially in limed soils, since the application of Ca-rich compounds has been found to be generally efficient for Pb immobilization (Kumpiene et al. 2008; Romero-Freire et al. 2015). However, As solubility increased in carbonate soils and with higher organic carbon content, so that the highest As soluble concentrations between treatments were found under GVS and MVS. The increase in As mobility after the application of liming treatments and the increase of soil pH has been reported by previous studies (Simón et al. 2010; Romero-Freire et al. 2014). Also, an increase in As solubility induced by organic amendment, as in vermicompost treatments in our case, has also been pointed out (Karczewska et al. 2017; Aftabtalab et al. 2022). This could be associated with As competition with organic matter for sorption sites and metal oxide surfaces, which may represent a primary mechanism for As release and thus increasing its mobility (Redman et al. 2002; Bauer and Blodau 2006). On the other hand, the behavior of Zn and Cu solubility under treatments was very similar in both, and directly related to their strong negative correlation with pH (Wang et al. 2013; Laurent et al. 2020), showing Spearman’s correlation coefficients with pH of -0.933 and -0.833 respectively (n = 9). Thus, soils rich in CaCO3 content were the most effective in decreasing their soluble concentrations, which were those amended with marble (MS, MVS) and gypsum (GS, GVS), the treatments with a higher pH. This is in accordance with previous studies that highlighted the effectiveness of soils rich in CaCO3 in retaining these elements (Aguilar et al. 2004). On the contrary, the highest water-soluble concentrations for Cu and Zn were found under BS and BVS treatments, where the pH was neutralized in a lesser extent, and especially in the case of Zn (Fig. S2), which reaches the highest mobility under acidic conditions and it is considered to present a higher mobility in soil with respect to Cu (Rocco et al. 2018; García-Carmona et al. 2019).
EDTA-extractable fraction of PTEs is also used to assess their bioavailability in soils (Kidd et al. 2007; García-Carmona et al. 2019). Unlike water-soluble fraction, EDTA-extractable fraction is regarded by different authors as a more reliable predictor of long-term availability of these elements in soils (Hurdebise et al. 2015). Moreover, among the different extraction methods frequently used for evaluating the bioavailable fraction of PTEs in soil (e.g. DTPA, EDTA, CaCl2, and NaNO3), EDTA was selected since it is considered more suitable for acidic soils (Hammer and Keller 2002; Feng et al. 2005), and it has a stronger extraction capacity than other extraction methods (Quevauviller et al. 1998; Han et al. 2020) This can be related to the EDTA high extraction potential, which may provide the maximum element extractability and, thus, indicate the higher levels of metal mobility (Labanowski et al. 2008). Furthermore, chelating agents such as EDTA are considered a more accurate indicator of metal availability to plants, as they can more effectively remove soluble metal–organic complexes that are potentially bioavailable (Bolan et al. 2008).
EDTA-extractable fraction in US showed the lowest concentrations for all PTEs except for Pb, where the highest concentration was found followed by RS (Table 4). These values may be the result of an overestimation caused by the EDTA’s significant binding capacity for Pb and its great efficiency in mobilizing this element from the soil (Shen et al. 2002; Santos et al. 2010). In addition, high Pb EDTA-extractable concentrations found in US and RS may be enhanced by the high organic carbon content in these soils coupled with EDTA capacity to effectively displace organically-bound fractions of metals present in soil through formation of strong chelates (Elliott and Shastri 1999; Nakamaru and Martín-Peinado 2017). The specific increase in Pb availability in soil due to the application of organic compounds has been previously proven (Angin et al. 2008). On the other hand, in PS the EDTA-extractable fraction was significantly the lowest for Pb, and for As and Cu were lower than in the amended soil treatments. Regarding the treatments applied to the amended soils, BS and BVS showed higher concentrations of Pb extractable with EDTA, driven by the higher organic carbon content in these treatments, while marble and gypsum treatments did so for As. Overall, vermicompost treatments showed higher concentrations of EDTA-extractable pollutants, which can be attributed to a positive correlation between organic matter content in soil and EDTA-extractable contents of PTEs, as previously reported by Zeng et al. 2011.
Species richness and vegetation cover after remediation measures
The presence of vegetation in the studied soils eighteen months after the application of the treatments was evaluated compared to that present in the US. In this soil, a mean of about 12 different plant species was found growing on it (Fig. 2a). Compared to US, species richness was slightly lower in RS, which could be related to the higher soluble concentrations found for the elements with highest mobility such as Zn and Cu, which in concentrations that exceed those required as nutrients may cause phytotoxicity to specific plant species (Nagajyoti et al. 2010). The high mobility of these elements could be, therefore, the main factor influencing the decrease in vegetation diversity and even, as in the case of PS, the responsible for the complete lack of vegetation on this soil, where the highest mobility for these elements was reached. In this regard, García-Carmona et al. (2019) reported that the absence of vegetation in the soils that remain polluted in the studied area is mainly due to the high water-soluble concentration of Cu and Zn, rather than the high total concentration of As and Pb. Regarding the treated soils, species richness was half in the treatments with vermicompost addition compared to US, while for those without vermicompost it represented about just one-third. Meanwhile, both US and RS were totally covered by vegetation unlike PS, where vegetation growth was completely inhibited (Fig. 2b). Under vermicompost treatments, vegetation cover was slightly lower if compared to US and RS, while for the treatments without vermicompost addition the vegetation cover represented less than half of that appearing when vermicompost was added, driven by the lower organic carbon content on these treatments. However, in general, all the treatments applied on PS resulted in enhanced soil properties and decreased soil toxicity, enabling the growth of vegetation to different extents, with the vermicompost treatments showing the greatest vegetation cover and species richness. Thus, vermicompost treatments were the most effective ones in terms of plant recolonization and development on the polluted soils, emphasizing that the addition of organic amendments in bare polluted soils is essential to facilitate the reestablishment and recolonization by the pioneer vegetation (Wong 2003). This vegetation development found on treated soils represents the spontaneous and natural response of the local vegetation to the changes produced in the soil by the amendments after eighteen months, so that the vegetation development in the treated soils could be expected to be similar to that found in the RS in the medium term. This could be assisted not only by the changes in soil properties induced by the treatments, but also by vegetation recolonization itself, since its presence produces many beneficial effects at the rhizosphere level and on the surrounding polluted soil. These can range from stimulation of microbial activity, improved aeration and stabilization of the soil to the reduction of PTEs concentrations and their stabilization (Erickson 1997; Davis et al. 2002). Moreover, the very presence of vegetation may also facilitate other plant species to subsequently colonize the area through various mechanisms that reduce the potential transfer of elements from the soil to the plants, as the modification of rhizosphere pH or the exudation into the soil of organic acids that bind pollutants (Rutkowska et al. 2020).
a Mean plant species richness present in each of the soils and treatments studied. b Total vegetation cover (%), and Spergularia rubra (Sr) and Lamarckia aurea (La) specific cover in each of the soils and treatments studied. US: Unpolluted soil; RS: Recovered soil; PS: Polluted soil; BS: Biopile; BVS: Biopile + vermicompost; GS: Gypsum; GSV: Gypsum + vermicompost; MS: Marble; MVS: Marble + vermicompost. Bars with different letters are significantly different according to Kruskal–Wallis test (P < 0.05)
Frequently, in soils polluted by PTEs, their toxicity restricts the growth of all but the most tolerant plant species (Wong 2003). In our case, polluted soils did not show vegetation cover at all. However, eighteen months after the application of the amendments, mainly two species highly tolerant to the present PTEs concentrations appeared in all the treated soils: the herbaceous plants Spergularia rubra (L.) J. Presl & C. Presl and Lamarckia aurea (L.) Moench, being S. rubra the one with higher colonization percentage in all treatments (Fig. 2b), present in about one third of the surface in treatments without vermicompost and in more than two thirds in those with vermicompost. Both species are frequently found in the soils polluted after the Aznalcóllar spill, particularly in those areas where PTEs concentrations remain higher, with acidic pH and high EC, and their presence being restricted mostly to them (Madejón et al. 2006; Montiel-Rozas et al. 2016; García-Carmona et al. 2019). In this study, both species were dominant in the treated polluted soils where conditions (i.e., PTEs concentrations) remain more restrictive for vegetation growth. On the contrary, their presence was barely detected in US and RS, where a higher number of less pollution-tolerant species are present.
PTEs concentration and accumulation in plants
To fully evaluate the success of remediation strategies implemented on polluted soils, the determination of PTEs fractions in soil may not be sufficient to accurately predict the PTEs transfer risk to plants, since plant uptake not always correlates with them (Proto et al. 2023). Therefore, for a more accurate assessment of revegetation success, plant-based approaches should be used, either by carrying out plant bioassays or measuring PTEs concentrations in spontaneously growing vegetation in remediated soils, as this is a more reliable indicator of PTEs transfer potential in soils (Milton et al. 2002; Proto et al. 2023).
According to the predominant presence of S. rubra and L. aurea in the treated soils, their PTEs accumulation capacity was assessed (Table 5). Both species were able to accumulate high concentrations of PTEs both in their shoots and roots, which in most cases correlated significantly with the total and bioavailable concentration of the PTEs in the soil. In contrast, no direct correlation was observed with the soluble fraction of PTEs in soil, except for Zn concentrations (Table S2). In general, the concentrations measured in L. aurea were higher than in S. rubra for all elements except for Zn, which accumulated at a higher rate in S. rubra, and especially in the shoots of this species. The high Zn accumulation in S. rubra, and especially in its shoots, was previously reported by other authors (El Berkaoui et al. 2021). Meanwhile, L. aurea showed elevated PTEs accumulation for the other studied elements, highlighting the concentrations of Pb and As measured particularly in its roots. This confirms the high ability of this species to accumulate multiple PTEs, both in its shoots and roots, and an exceptional high capacity to accumulate specific elements in roots such as Pb (Condori 2004; Midhat et al. 2017).
In relation to plant indexes, the bioaccumulation factor (BAF) was calculated considering the PTEs bioavailable (EDTA-extractable) fraction in soil, since this fraction is considered more precise for this end (Losfeld et al. 2015). This is based in the fact that the danger of PTEs lies in their solubility and bioavailability rather than in their total concentrations, since they better represent the fractions that are available for uptake by living organisms and that can spread within the soil (Marguí et al. 2007; García-Carmona et al. 2019). Moreover, total metal concentrations in soil are considered to correlate poorly with metal concentrations in plant tissues, since the amount of an element that is actually available to plants is different from that assessed by strong acid digestions (Buscaroli 2017). In this sense, BAF pointed out that S. rubra and L. aurea have a significant capability for accumulating Pb and As, both in their shoots and in their roots (Fig. 3). According to the values obtained for these elements in both parts (BAF > 10), we could consider that these two species act as hyperaccumulators of Pb and As (Rutkowska et al. 2020). Comparing these species to each other in terms of Pb and As accumulation, L. aurea had greater BAF than S. rubra, especially in the roots, highlighting that L. aurea is particularly tolerant to high levels of these elements, showing a higher ability to accumulate them in both the shoots and roots without sustaining toxicity, as remarked by previous studies (Midhat et al. 2017). Besides, the high values observed for Pb and As BAF in roots of L. aurea suggest that the translocation of these elements from soil to root was considerably high, so that L. aurea roots act as sink for Pb and As accumulation (Ng et al. 2016).
According to Zn, BAF measured for this element in both species was greater than one (Fig. 3), although in a significantly lower degree than for Pb and As. Zn low accumulation (BAF < 1) in different grass plants was observed even at increasing concentrations (Andrejić et at. 2018; Grassi et al. 2020), and even plants restricting Zn uptake at higher concentrations in the soil (Andrejić et al. 2018). Therefore, we could consider that although not at the same scale than Pb and As, the BAF > 1 obtained for the studied species for Zn indicates that there is a considerable transfer of this element into the plant. Comparing both species, the higher accumulation of Zn showed by S. rubra (Figs. 3 and 4) indicates that it is highly tolerant to this element (Gutiérrez Ginés 2013), which could be one of the reasons of the higher abundance of this species compared to L. aurea (Fig. 2b). In terms of Cu, neither S. rubra nor L. aurea showed a significant accumulation capacity for this element (BAF < 1), which only slightly accumulated on L. aurea roots, and which is in accordance with the observed by García-Carmona et al. (2019) in plants growing in residual polluted soils in the same area. The low Cu accumulation capacity in grass plants (BAF < 1) was also reported in other studies (Bhatti et al. 2016; Satpathy et al. 2014).
The translocation factor (TF) calculated for the two selected species was lower than one in all cases, except very slightly for Zn in S. rubra, so that elements migration from the roots to the shoots of these species is not substantial (Fig. 4). Nevertheless, TF in S. rubra for all elements was approximately two times higher than in L. aurea, showing that PTEs migration to S. rubra shoots is higher than that in L. aurea. Thus, the high capacity for pollutants concentration shown by L. aurea in its roots could reveal the mechanism that allows this species to thrive in the most heavily polluted soils and, consequently, be used as an accurate bioindicator of trace element availability in soils in this area, which is in accordance with Burgos et al. (2008).
The natural settling of these two species, growing in the remediated soils after eighteen months, are good indicators of the success of the remediation treatments used (Álvarez-Rogel et al. 2021). In addition, although they were tolerant to the PTEs present in the soil and can accumulate some of them (BAF > 1 for As, Pb and Zn), the absence of elements translocation from roots to shoots in the studied plants indicate that the selected cost-effective remediation techniques used, over time, may follow with passive enhancement, by modification of soil conditions by colonizing plants and being the first step in facilitating the growth of other species less tolerant to the stress (Navarro-Cano et al. 2018).
Conclusions
The use of this novel cost-effective soil remediation strategy, implemented under field conditions for long-term soil remediation approach, has demonstrated significant improvements in key soil properties following treatments application, including elevated pH levels, augmented organic carbon content, and decreased salinity levels. Consequently, these treatments proved highly effective in fostering spontaneous vegetation colonization within severely degraded soil patches. Among the various remediation approaches, vermicompost amendment proved to be the most effective in promoting the vegetation recovery in the treated soils, leading to a greater diversity of plant species and increased vegetation cover in them.
The long-term field-based approach is crucial when evaluating the spontaneous recolonization of remediated soils by native vegetation, and the role of the actions implemented in achieving this. In this regard, two plant species relevant in the studied area and predominant in the treated soils after settling of treatments over time, Spergularia rubra and Lamarkia aurea, exhibited remarkable capacity in accumulating Pb and As, particularly in their root systems, indicating their high tolerance to the elevated pollutant concentrations prevalent in the study area. This points their role as key species, as they not only serve as pioneers in recolonizing the degraded soils but also facilitate the subsequent recolonization by other species less tolerant to the high pollution levels present in the studied area. Therefore, the success of the remediation strategy assessed may be promoted by the early recolonization of the soil by the high pollution-tolerant species, since their presence enhance further evolution in the soil physical, biological, and chemical conditions.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Adak T, Singha A, Kumar K, Shukla SK, Singh A, Singh VK (2014) Soil organic carbon, dehydrogenase activity, nutrient availability and leaf nutrient content as affected by organic and inorganic source of nutrient in mango orchard soil. J Soil Sci Plant Nutr 14:394–406. https://doi.org/10.4067/S0718-95162014005000031
Adhikari K, Hartemink AE (2016) Linking soils to ecosystem services—a global review. Geoderma 262:101–111. https://doi.org/10.1016/j.geoderma.2015.08.009
Adriano DC, Wenzel WW, Vangronsveld J, Bolan NS (2004) Role of assisted natural remediation in environmental cleanup. Geoderma 122:121–142. https://doi.org/10.1016/j.geoderma.2004.01.003
Aftabtalab A, Rinklebe J, Shaheen SM, Niazi NK, Moreno-Jiménez E, Schaller J, Knorr KH (2022) Review on the interactions of arsenic, iron (oxy)(hydr) oxides, and dissolved organic matter in soils, sediments, and groundwater in a ternary system. Chemosphere 286:131790. https://doi.org/10.1016/j.chemosphere.2021.131790
Aguilar J, Bouza P, Dorronsoro C, Fernández E, Fernández J, García I, Martín-Peinado FJ, Simón M (2004) Application of remediation techniques for immobilization of metals in soils contaminated by a pyrite tailing spill in Spain. Soil Use Manage 20:451–453. https://doi.org/10.1111/j.1475-2743.2004.tb00396.x
Alibrahim ZO, Williams CD (2016) Assessment of bioavailability of some potential toxic metals in mining-affected soils using EDTA extraction and principle component analysis (PCA) approach, Derbyshire, UK. Interdiscip J Chem 1:58–65. https://doi.org/10.15761/IJC.1000110
Álvarez-Rogel J, Peñalver-Alcalá A, Jiménez-Cárceles FJ, Tercero MC, González-Alcaraz MN (2021) Evidence supporting the value of spontaneous vegetation for phytomanagement of soil ecosystem functions in abandoned metal(loid) mine tailings. CATENA 201:105191. https://doi.org/10.1016/j.catena.2021.105191
Andrejić G, Gajić G, Prica M, Dželetović Ž, Rakić T (2018) Zinc accumulation, photosynthetic gas exchange, and chlorophyll a fluorescence in Zn-stressed Miscanthus× giganteus plants. Photosynthetica 56:1249–1258. https://doi.org/10.1007/s11099-018-0827-3
Angin I, Turan M, Ketterings QM, Cakici A (2008) Humic acid addition enhances B and Pb phytoextraction by vetiver grass (Vetiveria zizanioides (L.) Nash). Water Air Soil Pollut 188:335–343. https://doi.org/10.1007/s11270-007-9548-0
Anning AK, Akoto R (2018) Assisted phytoremediation of heavy metal contaminated soil from a mined site with Typha latifolia and Chrysopogon zizanioides. Ecotoxicol Environ Saf 148:97–104. https://doi.org/10.1016/j.ecoenv.2017.10.014
Arienzo M, Adamo P, Cozzolino V (2004) The potential of Lolium perenne for revegetation of contaminated soil from a metallurgical site. Sci Total Environ 319:13–25. https://doi.org/10.1016/S0048-9697(03)00435-2
Bagherifam S, Brown TC, Fellows CM, Naidu R (2019) Bioavailability of arsenic and antimony in terrestrial ecosystems: a review. Pedosphere 29:681–720. https://doi.org/10.1016/S1002-0160(19)60843-X
Baker A, Ernst W, Van der Ent A, Malaisse F, Ginocchio R (2010) Metallophytes: the unique biological resource, its ecology and conservational status in Europe, central Africa and Latin America. In: Batty L, Hallberg K (eds) Ecology of industrial pollution. Cambridge University Press, Cambridge, pp 7–40. https://doi.org/10.1017/CBO9780511805561.003
Balasoiu CF, Zagury GJ, Deschenes L (2001) Partitioning and speciation of chromium, copper, and arsenic in CCA-contaminated soils: influence of soil composition. Sci Total Environ 280:239–255. https://doi.org/10.1016/S0048-9697(01)00833-6
Barahona E (1984) Determinación de carbonatos totales y caliza activa. Grupo de trabajo de normalización de métodos analíticos. In: I Congreso Nacional de la Ciencia del Suelo. Sociedad Española de la Ciencia del Suelo, Madrid, pp 53–67
Bauer M, Blodau C (2006) Mobilization of arsenic by dissolved organic matter from iron oxides, soils and sediments. Sci Total Environ 354:179–190. https://doi.org/10.1016/j.scitotenv.2005.01.027
Bernal MP, Clemente R, Walker DJ (2007) The role of organic amendments in the bioremediation of heavy metal-polluted soils. In: Gore RW (ed) Environmental research at the leading edge. Nova Science Publishers, Inc., New York, EEUU
Bhatti SS, Kumar V, Singh N, Sambyal V, Singh J, Katnoria JK, Nagpal AK (2016) Physico-chemical properties and heavy metal contents of soils and kharif crops of Punjab, India. Procedia Environ Sci 35:801–808. https://doi.org/10.1016/j.proenv.2016.07.096
BOJA (Boletín Oficial de la Junta de Andalucía) (2015) Decreto 18/2015, de 27 de enero, por el que se aprueba el reglamento que regula el régimen aplicable a los suelos contaminados. Consejería de Medio Ambiente y Ordenación del Territorio. Junta de Andalucía, España, 28–64. https://www.juntadeandalucia.es/boja/2015/38/3. Accessed Dec 2023
Boi ME, Cappai G, de Giudici G, Medas D, Piredda M, Porceddu M, Bacchetta G (2021) Ex situ phytoremediation trial of Sardinian mine waste using a pioneer plant species. Environ Sci Pollut Res 28:55736–55753. https://doi.org/10.1007/s11356-021-14710-y
Bolan NS, Ko BG, Anderson CWN, Vogeler I, Mahimairaja S, Naidu R (2008) Manipulating bioavailability to manage remediation of metal-contaminated soils. Dev Soil Sci 32:657–678. https://doi.org/10.1016/S0166-2481(07)32027-8
Bolan NS, Park JH, Robinson B, Naidu R, Huh KY (2011) Chapter four - phytostabilization: a green approach to contaminant containment. Adv Agron 112:145–204. https://doi.org/10.1016/B978-0-12-385538-1.00004-4
Burgos P, Pérez-de-Mora A, Madejón P, Cabrera F, Madejón E (2008) Trace elements in wild grasses: a phytoavailability study on a remediated field. Environ Geochem Health 30:109–114. https://doi.org/10.1007/s10653-008-9135-3
Buscaroli A (2017) An overview of indexes to evaluate terrestrial plants for phytoremediation purposes. Ecol Indic 82:367–380. https://doi.org/10.1016/j.ecolind.2017.07.003
Cabrera F, Clemente L, Cordón R, Hurtado MD, López R, Madejón P, Marañón T, Moreno F, Murillo JM, Nagel I (2005) Effect of remediation on trace metal pollution in soils of Guadiamar river valley. In: Del Valls TA, Blasco J (eds) Integrated assessment and management of the ecosystems affected by the Aznalcóllar mining spill (SW, Spain). Cátedra UNESCO/Unitwin, Cádiz, pp. 33–40
Cabrera F, Ariza JL, Madejón P, Madejón E, Murillo JM (2008) Mercury and other trace elements in soils affected by the mine tailing spill in Aznalcóllar (SW Spain). Sci Total Environ 390:311–322. https://doi.org/10.1016/j.scitotenv.2007.10.002
Clemente R, Pardo T, Madejón P, Madejón E, Bernal MP (2015) Food byproducts as amendments in trace elements contaminated soils. Food Res Int 73:176–189. https://doi.org/10.1016/j.foodres.2015.03.040
Condori EF (2004) Estudio de la vegetación espontánea de un suelo contaminado con elementos traza en proceso de biorecuperación. CSIC-IRNAS, Sevilla
Davis LC, Castro-Diaz S, Zhang Q, Erickson LE (2002) Benefits of vegetation for soils with organic contaminants. Crit Rev Plant Sci 21:457–491. https://doi.org/10.1080/0735-260291044322
Dermont G, Bergeron M, Mercier G, Richer-Laflèche M (2008) Metal-contaminated soils: remediation practices and treatment technologies. Pract Period Hazard, Toxic, Radioact Waste Manag 12:188–209. https://doi.org/10.1061/(ASCE)1090-025X(2008)12:3(188)
Egli M, Sartori G, Mirabella A, Giaccai D, Favilli F, Scherrer D, Krebs R, Delbos E (2010) The influence of weathering and organic matter on heavy metals lability in silicatic, Alpine soils. Sci Total Environ 408:931–946. https://doi.org/10.1016/j.scitotenv.2009.10.005
El Berkaoui M, El Adnani M, Hakkou R, Ouhammou A, Bendaou N, Smouni A (2021) Phytostabilization of phosphate mine wastes used as a store-and-release cover to control acid mine drainage in a semiarid climate. Plants 10:900. https://doi.org/10.3390/plants10050900
Elliott HA, Shastri NL (1999) Extractive decontamination of metal-polluted soils using oxalate. Water Air Soil Pollut 110:335–346. https://doi.org/10.1023/A:1005067404259
Erickson LE (1997) An overview of research on the beneficial effects of vegetation in contaminated soil. Ann N Y Acad Sci 829:30–35. https://doi.org/10.1111/j.1749-6632.1997.tb48563.x
FAO and ITPS (2015) Status of the World’s Soil Resources (SWSR) – Main report. Food and Agriculture Organization of the United Nations and Intergovernmental Technical Panel on Soils, Rome, Italy. FAO, p 650. http://www.fao.org/3/a-i5199e.pdf. Accessed Dec 2023
Feng MH, Shan XQ, Zhang S, Wen B (2005) A comparison of the rhizosphere-based method with DTPA, EDTA, CaCl2, and NaNO3 extraction methods for prediction of bioavailability of metals in soil to barley. Environ Pollut 137:231–240. https://doi.org/10.1016/j.envpol.2005.02.003
Fernández P, Sommer I, Cram S, Rosas I, Gutiérrez M (2005) The influence of water-soluble As (III) and As (V) on dehydrogenase activity in soils affected by mine tailings. Sci Total Environ 348:231–243. https://doi.org/10.1016/j.scitotenv.2004.12.065
Fernández-Caliani JC, Barba-Brioso C (2010) Metal immobilization in hazardous contaminated minesoils after marble slurry waste application. A field assessment at the Tharsis mining district (Spain). J Hazard Mater 181:817–826. https://doi.org/10.1016/j.jhazmat.2010.05.087
Gall JE, Boyd RS, Rajakaruna N (2015) Transfer of heavy metals through terrestrial food webs: a review. Environ Monit Assess 187:201. https://doi.org/10.1007/s10661-015-4436-3
Gangloff S, Stille P, Pierret MC, Weber T, Chabaux F (2014) Characterization and evolution of dissolved organic matter in acidic forest soil and its impact on the mobility of major and trace elements (case of the Strengbach watershed). Geochim Cosmochim Acta 130:21–41. https://doi.org/10.1016/j.gca.2013.12.033
García-Carmona M, Romero-Freire A, Sierra-Aragón M, Martínez-Garzón FJ, Martín Peinado FJ (2017) Evaluation of remediation techniques in soils affected by residual contamination with heavy metals and arsenic. J Environ Manage 191:228–236. https://doi.org/10.1016/j.jenvman.2016.12.041
García-Carmona M, García-Robles H, Torrano CT, Fernández-Ondoño E, Lorite J, Sierra-Aragón M, Martín-Peinado FJ (2019) Residual pollution and vegetation distribution in amended soils 20 years after a pyrite mine tailings spill (Aznalcóllar, Spain). Sci Total Environ 650:933–940. https://doi.org/10.1016/j.scitotenv.2018.09.092
García-Robles H, Melloni EG, Navarro FB, Martín-Peinado FJ, Lorite J (2022) Gypsum mining spoil improves plant emergence and growth in soils polluted with potentially harmful elements. Plant Soil 481:315–329. https://doi.org/10.1007/s11104-022-05639-3
Ghosh AK, Bhattacharyya P, Pal R (2004) Effect of arsenic contamination on microbial biomass and its activities in arsenic contaminated soils of Gangetic West Bengal, India. Environ Int 30:491–499. https://doi.org/10.1016/j.envint.2003.10.002
Gomez-Eyles JL, Sizmur T, Collins CD, Hodson ME (2011) Effects of biochar and the earthworm Eisenia fetida on the bioavailability of polycyclic aromatic hydrocarbons and potentially toxic elements. Environ Pollut 159:616–622. https://doi.org/10.1016/j.envpol.2010.09.037
González V, García I, del Moral F, Simón M (2012) Effectiveness of amendments on the spread and phytotoxicity of contaminants in metal-arsenic polluted soil. J Hazard Mater 205–206:72–80. https://doi.org/10.1016/j.jhazmat.2011.12.011
Grassi C, Cecchi S, Baldi A, Zanchi CA, Orlandini S, Pardini A, Napoli M (2020) Crop suitability assessment in remediation of Zn contaminated soil. Chemosphere 246:125706. https://doi.org/10.1016/j.chemosphere.2019.125706
Greyson J (2007) An economic instrument for zero waste, economic growth and sustainability. J Clean Prod 15:1382–1390. https://doi.org/10.1016/j.jclepro.2006.07.019
Grimalt JO, Ferrer M, Macpherson E (1999) The mine tailings accident in Aznalcóllar. Sci Total Environ 242:3–11. https://doi.org/10.1016/S0048-9697(99)00372-1
Gutiérrez Ginés MJ (2013) Responses shown by plant populations to real settings of polluted soils: an ecological approach to remediation. Universidad de Alcalá (Alcalá de Henares, Spain), pp 255. http://hdl.handle.net/10017/20346
Hammer D, Keller C (2002) Changes in the rhizosphere of metal-accumulating plants evidenced by chemical extractants. J Environ Qual 31:1561–1569. https://doi.org/10.2134/jeq2002.1561
Han HJ, Lee JU, Ko MS, Kim KW (2020) Comparison of five extraction methods for evaluating cadmium and zinc immobilization in soil. Environ Geochem Health 42:4203–4212. https://doi.org/10.1007/s10653-020-00650-y
Hurdebise Q, Tarayre C, Fischer C, Colinet G, Hiligsmann S, Delvigne F (2015) Determination of zinc, cadmium and lead bioavailability in contaminated soils at the single-cell level by a combination of whole-cell biosensors and flow cytometry. Sensors 15:8981–8999. https://doi.org/10.3390/s150408981
Igalavithana AD, Mahagamage MGY, Gajanayake P, Abeynayaka A, Gamaralalage PJD, Ohgaki M, Takenaka M, Fukai T, Itsubo N (2022) Microplastics and potentially toxic elements: potential human exposure pathways through agricultural lands and policy based countermeasures. Microplastics 1:102–120. https://doi.org/10.3390/microplastics1010007
Kabata-Pendias A (2010) Trace elements in soils and plants, 4th ed. CRC Press, Taylor & Francis Group, LLC, Boca Raton, p. 548. https://doi.org/10.1201/b10158
Karczewska A, Gałka B, Dradrach A, Lewińska K, Mołczan M, Cuske M, Gersztyn L, Litak K (2017) Solubility of arsenic and its uptake by ryegrass from polluted soils amended with organic matter. J Geochem Explor 182:193–200. https://doi.org/10.1016/j.gexplo.2016.11.020
Kidd PS, Domínguez-Rodríguez MJ, Díez J, Monterroso C (2007) Bioavailability and plant accumulation of heavy metals and phosphorus in agricultural soils amended by long-term application of sewage sludge. Chemosphere 66:1458–1467. https://doi.org/10.1016/j.chemosphere.2006.09.007
Kumpiene J, Lagerkvist A, Maurice C (2008) Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments–a review. Waste Manage 28:215–225. https://doi.org/10.1016/j.wasman.2006.12.012
Labanowski J, Monna F, Bermond A, Cambier P, Fernández C, Lamy I, Van Oort F (2008) Kinetic extractions to assess mobilization of Zn, Pb, Cu, and Cd in a metal-contaminated soil: EDTA vs. citrate. Environ Pollut 152:693–701. https://doi.org/10.1016/j.envpol.2007.06.054
Laurent C, Bravin MN, Crouzet O, Pelosi C, Tillard E, Lecomte P, Lamy I (2020) Increased soil pH and dissolved organic matter after a decade of organic fertilizer application mitigates copper and zinc availability despite contamination. Sci Total Environ 709:135927. https://doi.org/10.1016/j.scitotenv.2019.135927
Li G, Khan S, Ibrahim M, Sun TR, Tang JF, Cotner JB, Xu YY (2018) Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium. J Hazard Mater 348:100–108. https://doi.org/10.1016/j.jhazmat.2018.01.031
Liu L, Li W, Song W, Guo M (2018) Remediation techniques for heavy metal-contaminated soils: principles and applicability. Sci Total Environ 633:206–219. https://doi.org/10.1016/j.scitotenv.2018.03.161
Lorente-Casalini O, García-Carmona M, Pastor-Jáuregui R, Martín-Peinado FJ (2021) Assessment of biopiles treatment on polluted soils by the use of Eisenia andrei bioassay. Environ Pollut 275:116642. https://doi.org/10.1016/j.envpol.2021.116642
Losfeld G, L’Huillier L, Fogliani B, McCoy S, Grison C, Jaffré T (2015) Leaf-age and soil-plant relationships: key factors for reporting trace-elements hyperaccumulation by plants and design applications. Environ Sci Pollut Res 22:5620–5632. https://doi.org/10.1007/s11356-014-3445-z
Madejón E, Pérez-de-Mora A, Felipe E, Burgos P, Cabrera F (2006) Soil amendments reduce trace element solubility in a contaminated soil and allow regrowth of natural vegetation. Environ Pollut 139:40–52. https://doi.org/10.1016/j.envpol.2005.04.034
Madejón P, Domínguez MT, Madejón E, Cabrera F, Marañón T, Murillo-Carpio JM (2018) Soil-plant relationships and contamination by trace elements: a review of twenty years of experimentation and monitoring after the Aznalcóllar (SW Spain) mine accident. Sci Total Environ 625:50–63. https://doi.org/10.1016/j.scitotenv.2017.12.277
MAPA (1994) Métodos Oficiales de Análisis. Tomo 3. Ministerio de Agricultura, Pesca y Alimentación. Secretaría General: Madrid, p 532
Marguí E, Queralt I, Carvalho ML, Hidalgo M (2007) Assessment of metal availability to vegetation (Betula pendula) in Pb-Zn ore concentrate residues with different features. Environ Pollut 145:179–184. https://doi.org/10.1016/j.envpol.2006.03.028
McLaren RG, Swift RS, Williams JC (1981) The adsorption of copper by soil materials at low equilibrium solution concentrations. Eur J Soil Sci 32:247–256. https://doi.org/10.1111/j.1365-2389.1981.tb01704.x
Midhat L, Ouazzani N, Esshaimi M, Ouhammou A, Mandi L (2017) Assessment of heavy metals accumulation by spontaneous vegetation: screening for new accumulator plant species grown in Kettara mine-Marrakech, Southern Morocco. Int J Phytoremediation 19:191–198. https://doi.org/10.1080/15226514.2016.1207604
Milton A, Johnson MS, Cooke JA (2002) Lead within ecosystems on metalliferous mine tailings in Wales and Ireland. Sci Total Environ 299:177–190. https://doi.org/10.1016/S0048-9697(02)00253-X
Montiel-Rozas MM, López-García A, Kjøller R, Madejón E, Rosendahl S (2016) Organic amendments increase phylogenetic diversity of arbuscular mycorrhizal fungi in acid soil contaminated by trace elements. Mycorrhiza 26:575–585. https://doi.org/10.1007/s00572-016-0694-3
Muyessar T, Linsheng Y (2016) Trace elements contamination and human health risk assessment in drinking water from the agricultural and pastoral areas of Bay County, Xinjiang, China. Int J Environ Res Public Health 13:938. https://doi.org/10.3390/ijerph13100938
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216. https://doi.org/10.1007/s10311-010-0297-8
Nakamaru YM, Martín-Peinado FJ (2017) Effect of soil organic matter on antimony bioavailability after the remediation process. Environ Pollut 228:425–432. https://doi.org/10.1016/j.envpol.2017.05.042
Navarro-Cano JA, Verdú M, Goberna M (2018) Trait-based selection of nurse plants to restore ecosystem functions in mine tailings. J Appl Ecol 55:1195–1206. https://doi.org/10.1111/1365-2664.13094
Ng CC, Law SH, Amru NB, Motior MR, Radzi BA (2016) Phyto-assessment of soil heavy metal accumulation in tropical grasses. J Anim Plant Sci 26:686–696. https://doi.org/10.5281/zenodo.1019169
Nirola R, Megharaj M, Beecham S, Aryal R, Thavamani P, Vankateswarlu K, Saint C (2016) Remediation of metalliferous mines, revegetation challenges and emerging prospects in semi-arid and arid conditions. Environ Sci Pollut Res 23:20131–20150. https://doi.org/10.1007/s11356-016-7372-z
Park JH, Lamb D, Paneerselvam P, Choppala G, Bolan N, Chung JW (2011) Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J Hazard Mater 185:549–574. https://doi.org/10.1016/j.jhazmat.2010.09.082
Pérez-de-Mora A, Madejón E, Burgos P, Cabrera F (2006) Trace element availability and plant growth in a mine-spill contaminated soil under assisted natural remediation I. Soils. Sci Total Environ 363:28–37. https://doi.org/10.1016/j.scitotenv.2005.10.015
Pérez-de-Mora A, Burgos P, Cabrera F, Madejón E (2007) “In situ” amendments and revegetation reduce trace element leaching in a contaminated soil. Water Air Soil Poll 185:209–222. https://doi.org/10.1007/s11270-007-9443-8
Pietzsch N, Ribeiro JLD, de Medeiros JF (2017) Benefits, challenges and critical factors of success for Zero Waste: A systematic literature review. Waste Manag 67:324–353. https://doi.org/10.1016/j.wasman.2017.05.004
Pilon-Smits E (2005) Phytoremediation. Annu Rev Plant Biol 56:15–39. https://doi.org/10.1146/annurev.arplant.56.032604.144214
Poschenrieder C, Bech J, Llugany M, Pace A, Fenés E, Barceló J (2001) Copper in plant species in a copper gradient in Catalonia (North East Spain) and their potential for phytoremediation. Plant Soil 230:247–256. https://doi.org/10.1023/A:1010374732486
Proto M, Newsome L, Jensen E, Courtney R (2023) Geochemical analyses of metal (loid) fractions do not predict plant uptake behavior: are plant bioassays better tools to predict mine rehabilitation success? Sci Total Environ 861:160679. https://doi.org/10.1016/j.scitotenv.2022.160679
Quevauviller P, Lachica M, Barahona E, Gomez A, Rauret G, Ure A, Muntau H (1998) Certified reference material for the quality control of EDTA-and DTPA-extractable trace metal contents in calcareous soil (CRM 600). Fresenius J Anal Chem 360:505–511. https://doi.org/10.1007/s002160050750
Rahman Z, Singh VP (2019) The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: an overview. Environ Monit Assess 191:419. https://doi.org/10.1007/s10661-019-7528-7
Raklami A, Tahiri AI, Bechtaoui N, Pajuelo E, Baslam M, Meddich A, Oufdou K (2021) Restoring the plant productivity of heavy metal-contaminated soil using phosphate sludge, marble waste, and beneficial microorganisms. J Environ Sci 99:210–221. https://doi.org/10.1016/j.jes.2020.06.032
Rayed A, Omrane B, Chokri S, Mohamed-Amine K, Abdeliazim-Mustafa M (2019) Effects of incorporation of marble powder obtained by recycling waste sludge and limestone powder on rheology, compressive strength, and durability of self-compacting concrete. Adv Mater Sci Eng 2019. https://doi.org/10.1155/2019/4609353
Redman AD, Macalady DL, Ahmann D (2002) Natural organic matter affects arsenic speciation and sorption onto hematite. Environ Sci Technol 36:2889–2896. https://doi.org/10.1021/es0112801
Rocco C, Agrelli D, Tafuro M, Caporale AG, Adamo P (2018) Assessing the bioavailability of potentially toxic elements in soil: a proposed approach. Ital J Agron 13:16–22. https://doi.org/10.4081/ija.2018.1348
Rodríguez-Jordá MP, Garrido F, García-González MT (2012) Effect of the addition of industrial by-products on Cu, Zn, Pb and As leachability in a mine sediment. J Hazard Mater 213–214:46–54. https://doi.org/10.1016/j.jhazmat.2012.01.049
Romero-Baena AJ, Barba-Brioso C, Ross A, González I, Aparicio P (2021) Mobility of potentially toxic elements in family garden soils of the Riotinto mining area. Appl Clay Sci 203:105999. https://doi.org/10.1016/j.clay.2021.105999
Romero-Freire A, Sierra-Aragón M, Ortiz-Bernad I, Martín-Peinado FJ (2014) Toxicity of arsenic in relation to soil properties: implications to regulatory purposes. J Soils Sediments 14:968–979. https://doi.org/10.1007/s11368-014-0845-0
Romero-Freire A, Martín-Peinado FJ, Van Gestel CAM (2015) Effect of soil properties on the toxicity of Pb: Assessment of the appropriateness of guideline values. J Hazard Mater 289:46–53. https://doi.org/10.1016/j.jhazmat.2015.02.034
Rutkowska B, Szulc W, Błaszczak E, Kazberuk W, Ptasiński D (2020) Restoration of marginal soils polluted with heavy metals to agricultural production. J Soil Water Conserv 75:610–616. https://doi.org/10.2489/jswc.2020.00215
Sah RN, Miller RO (1992) Spontaneous reaction for acid dissolution of biological tissue in closed vessels. Anal Chem 64:230–233. https://doi.org/10.1021/ac00026a026
Santos S, Costa CA, Duarte AC, Scherer HW, Schneider RJ, Esteves VI, Santos EB (2010) Influence of different organic amendments on the potential availability of metals from soil: a study on metal fractionation and extraction kinetics by EDTA. Chemosphere 78:389–396. https://doi.org/10.1016/j.chemosphere.2009.11.008
Sanz-Ramos M, Bladé E, Dolz J, Sánchez-Juny M (2022) Revisiting the Hydraulics of the Aznalcóllar mine disaster. Mine Water Environ 41:335–356. https://doi.org/10.1007/s10230-022-00863-w
Satpathy D, Reddy MV, Dhal SP (2014) Risk assessment of heavy metals contamination in paddy soil, plants, and grains (Oryza sativa L.) at the East Coast of India. BioMed Res Int 2014:545473. https://doi.org/10.1155/2014/545473
Sauvé S, Martínez CE, McBride M, Hendershot W (2000) Adsorption of free lead (Pb2+) by pedogenic oxides, ferrihydrite, and leaf compost. Soil Sci Soc Am 64:595–599. https://doi.org/10.2136/sssaj2000.642595x
Shen ZG, Li XD, Wang CC, Chen HM, Chua H (2002) Lead phytoextraction from contaminated soil with high-biomass plant species. J Environ Qual 31:1893–1900. https://doi.org/10.2134/jeq2002.1893
Shen Z, Yu Z, Xu L, Zhao Y, Yi S, Shen C, Wang Y, Li Y, Zuo W, Gu C, Shan Y, Bai Y (2022) Effects of vermicompost application on growth and heavy metal uptake of barley grown in mudflat salt-affected soils. Agronomy 12:1007. https://doi.org/10.3390/agronomy12051007
Sierra-Aragón M, Nakamaru YM, García-Carmona M, Martínez-Garzón FJ, Martín-Peinado FJ (2019) The role of organic amendment in soils affected by residual pollution of potentially harmful elements. Chemosphere 237:124549. https://doi.org/10.1016/j.chemosphere.2019.124549
Simon L (2014) Potentially harmful elements in agricultural soils. In: Bini C, Bech J (eds) PHEs, environment and human health. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-8965-3_3
Simón M, Ortíz I, García I, Fernández-Ondoño E, Fernández J, Dorronsoro C, Aguilar J (1999) Pollution of soils by the toxic spill of a pyrite mine (Aznalcóllar, Spain). Sci Total Environ 242:105–115. https://doi.org/10.1016/S0048-9697(99)00378-2
Simón M, Diez M, González V, García I, Martín-Peinado FJ, De Haro S (2010) Use of liming in the remediation of soils polluted by sulphide oxidation: a leaching-column study. J Hazard Mater 180:241–246. https://doi.org/10.1016/j.jhazmat.2010.04.020
Theodorsson-Norheim E (1986) Kruskal-Wallis test: BASIC computer program to perform nonparametric one-way analysis of variance and multiple comparisons on ranks of several independent samples. Comput Methods Programs Biomed 23:57–62. https://doi.org/10.1016/0169-2607(86)90081-7
Tyurin IV (1951) Analytical procedure for a comparative study of soil humus. Trudy Pochr Inst Dokuchaeva 33:5–21
Viehweger K (2014) How plants cope with heavy metals. Bot Stud 55:35. https://doi.org/10.1186/1999-3110-55-35
Wang AS, Angle JS, Chaney RL, Delorme TA, Reeves RD (2006) Soil pH effects on uptake of Cd and Zn by Thlaspi caerulescens. Plant Soil 281:325–337. https://doi.org/10.1007/s11104-005-4642-9
Wang C, Yang Z, Yuan X, Browne P, Chen L, Ji J (2013) The influences of soil properties on Cu and Zn availability in soil and their transfer to wheat (Triticum aestivum L.) in the Yangtze River delta region. China Geoderma 193:131–139. https://doi.org/10.1016/j.geoderma.2012.10.004
Wong JWC, Ip CM, Wong MH (1998) Acid-forming capacity of lead–zinc mine tailings and its implications for mine rehabilitation. Environ Geochem Health 20:149–155. https://doi.org/10.1023/A:1006589124204
Wong MH (2003) Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 50:775–780. https://doi.org/10.1016/S0045-6535(02)00232-1
Xiong J, Madejón P, Madejón E, Cabrera F (2015) Assisted natural remediation of a trace element-contaminated acid soil: an eight-year field study. Pedosphere 25:250–262. https://doi.org/10.1016/S1002-0160(15)60010-8
Yin Y, Impellitteri CA, You SJ, Allen HE (2002) The importance of organic matter distribution and extract soil: solution ratio on the desorption of heavy metals from soils. Sci Total Environ 287:107–119. https://doi.org/10.1016/S0048-9697(01)01000-2
Zacchini M, Pietrini F, Scarascia-Mugnozza G, Lori V, Pietrosanti L, Massacci A (2009) Metal tolerance, accumulation and translocation in poplar and willow clones treated with cadmium in hydroponics. Water Air Soil Pollut 197:23–34. https://doi.org/10.1007/s11270-008-9788-7
Zeng F, Ali S, Zhang H, Ouyang Y, Qiu B, Wu F, Zhang G (2011) The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environ Pollut 159:84–91. https://doi.org/10.1016/j.envpol.2010.09.019
Funding
Funding for open access publishing: Universidad de Granada/CBUA. This work was supported by the Research Project RTI 2018-094327-B-I00 and the predoctoral contract FPU-18/02901, both funded by the Spanish Ministry of Science, Innovation and Universities); the Junta de Andalusia Post-doctoral Operating Research Program FEDER 2014-2020 (ref. E-RNM-444-UGR20); the Tatiana-Pérez-de-Guzmán-el-Bueno Foundation PhD grant Programme 2016. This study was also carried out in the framework of the research projects: “Development of techniques for the ecological restoration of gypsum habitats, P11-RNM-7061” funded by Regional Government of Andalusia (Consejería de Economía, Innovación, Ciencia y Empleo, Junta de Andalucía, Proyectos de Excelencia, P11-RNM-7061) and “Study of the ecological basis for restoration of gypsum vegetation in the Ventas de Huelma and Escúzar quarries” funded by KNAUF GmbH Branch Spain (Project 3092, Fundación UGR-Empresa). We would like to thank Sandra Redondo Sánchez for her valuable help with the field and lab work. Funding for open access charge: Universidad de Granada / CBUA.
Author information
Authors and Affiliations
Contributions
Mario Paniagua-López: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing—Original Draft, Writing—Review & Editing, Visualization. Helena García-Robles: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing—Original Draft, Writing—Review & Editing, visualization. Antonio Aguilar-Garrido: Formal analysis, Investigation, Data curation, Writing—Review & Editing, Visualization. Ana Romero-Freire: Validation, Resources, Data curation, Writing—Review & Editing, Visualization, Supervision, Funding Acquisition. Juan Lorite: Conceptualization, Methodology, Validation, Resources, Writing—Review & Editing, Supervision, Project administration, Funding Acquisition. Manuel Sierra-Aragón: Conceptualization, Methodology, Validation, Resources, Writing—Original Draft, Writing—Review & Editing, Supervision, Project administration, Funding Acquisition.
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Michael Komárek.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paniagua-López, M., García-Robles, H., Aguilar-Garrido, A. et al. Vegetation establishment in soils polluted by heavy metal(loid)s after assisted natural remediation. Plant Soil 497, 257–275 (2024). https://doi.org/10.1007/s11104-024-06521-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-024-06521-0