Abstract
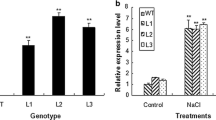
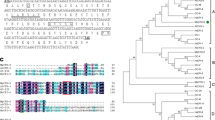
Two cysteine proteinase inhibitors (cystatins) from Arabidopsis thaliana, designated AtCYSa and AtCYSb, were characterized. Recombinant GST-AtCYSa and GST-AtCYSb were expressed in Escherichia coli and purified. They inhibit the catalytic activity of papain, which is generally taken as evidence for cysteine proteinase inhibitor function. Northern blot analyses showed that the expressions of AtCYSa and AtCYSb gene in Arabidopsis cells and seedlings were strongly induced by multiple abiotic stresses from high salt, drought, oxidant, and cold. Interestingly, the promoter region of AtCYSa gene contains a dehydration-responsive element (DRE) and an abscisic acid (ABA)-responsive element (ABRE), which identifies it as a DREB1A and AREB target gene. Under normal conditions, AtCYSa was expressed in 35S: DREB1A and 35S: AREB1 plants at a higher level than in WT plants, while AtCYSa gene was expressed in 35S: DREB2A plants at the same level as in WT plants. Under stress conditions (salt, drought and cold), AtCYSa was expressed more in all three transgenic plants than in WT plants. Over-expression of AtCYSa and AtCYSb in transgenic yeast and Arabidopsis plants increased the resistance to high salt, drought, oxidative, and cold stresses. Taken together, these data raise the possibility of using AtCYSa and AtCYSb to genetically improve environmental stresses tolerance in plants.









Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- ABRE:
-
ABA-responsive element
- AREB:
-
ABRE binding protein
- Cystatins:
-
Cysteine proteinase inhibitors
- DRE:
-
Dehydration-responsive element
- DREB:
-
DRE binding protein
- GST:
-
Glutathione S-transferase
- PCD:
-
Programmed cell death
- Pis:
-
Proteinase inhibitors
- PMSF:
-
Phenylmethylsulfonyl fluoride
- TCA:
-
Trichloroacetic acid
References
Abe K, Emori Y, Kondo H, Suzuki K, Arai S (1987) Molecular cloning of a cysteine proteinase inhibitors of rice (Oryzacystatin). Homology with animal cystatins and transient expression in the ripening process of rice seeds. J Biol Chem 262:16793–16797
Aoki H, Akaike T, Abe K, Kuroda M, Arai S, Okamura R, Negi A, Maeda H (1995) Antiviral effect of oryzacystatin, a proteinase inhibitor in rice, against herpes simplex virus type 1 in vitro and in vivo. Antimicrob Agents Chemother 39:846–849
Barrett AJ, Rawlings ND, Woessner JF (1998) Handbook of proteolytic enzymes. Academic Press, London
Belenghi B, Acconcia F, Trovato M, Perazzolli M, Bocedi A, Polticelli F, Ascenzi P, Delledonne M (2003) AtCYS1, a cystatin from Arabidopsis thaliana, suppresses hypersensitive cell death. FEBS Lett 270:2593–2604
Bolter CJ (1993) Methyl jasmonate induces papain inhibitor(s) in tomato leaves. Plant Physiol 103:1347–1353
Botella MA, Xu Y, Prabha TN, Zhao Y, Narasimhan ML, Wilson KA, Nielsen SS, Bressan RA, Hasegawa PM (1996) Differential expression of soybean cysteine proteinase inhibitor genes during development and in response to wounding and methyl jasmonate. Plant Physiol 112:1201–1210
Bray EA, Bailey-Serres JE, Weretilnyk E (2000) Responses to abiotic stresses. In: Gruissem W, Buchannan B, Jones R (eds) Biochemistry and molecular biology of plants. American Society of Plant Biologists, Rockville, MD, pp 158–1249
Callls J (1995) Regulation of protein degradation. Plant Cell 7:845–857
Christova PK, Christov NK, Imai R (2006) A cold inducible multidomain cystatin from winter wheat inhibits growth of the snow mold fungus Microdochium nivale. Planta 223:1207–1218
Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743
Davletova S, Rizhsky L, Liang H, Shengqiang Z, Oliver DJ, Coutu J, Shulaev V, Schlauch K, Mittler R (2005) Cytosolic ascorbate peroxidase 1 is a central component of the reactive oxygen gene network of Arabidopsis. Plant Cell 17:268–281
Diop NN, Kidric M, Repellin A, Gareil M, d’Arcy-Lameta A, Thi ATP, Zuily-Fodil Y (2004) A multicystatin is induced by drought-stress in cowpea (Vigna unguiculata (L.) Walp.) leaves. FEBS Lett 577:545–550
El Maarouf H, Zuily-Fodil Y, Gareil M, d’Arcy-Lameta A, Pham-Thi AT (1999) Enzymatic activity and gene expression under water stress of phospholipase D in two cultivars of Vigna unguiculata L. Walp. differing in drought tolerance. Plant Mol Biol 39:1257–1265
Fourcroy P, Vansuyt G, Kushnir S, Inzé D, Briat J (2004) Iron-regulated expression of a cytosolic ascorbate peroxidase encoded by the APX1 gene in Arabidopsis seedlings. Plant Physiol 134:605–613
Gaddour K, Vicente-Carbajosa J, Lara P, Isabel-Lamoneda I, Diaz I, Carbonero P (2001) A constitutive cystatin-encoding gene from barley (Icy) responds differentially to abiotic stimuli. Plant Mol Biol 45:599–608
Grudkowska M, Zagdańska B (2004) Multifunctional role of plant cysteine proteinases. Acta Biochim Pol 51:609–624
Gutiérrez-Campos R, Torres-Acosta JA, Saucedo-Arias LJ, Gómez-Lim MA (1999) The use of cysteine proteinase inhibitors to engineer resistance against potyviruses in transgenic tobacco plants. Nat Biotechnol 17:1223–1226
Gutiérrez-Campos R, Torres-Acosta JA, Pérez-Martinez JDJ, Gómez-Lim MA (2001) Pleiotropic effects in transgenic tobacco plants expressing oryzacystatin I gene. Hortscience 36:118–119
Hibbetts K, Hines B, Williams D (1999) An overview of proteinase inhibitors. J Vet Intern Med 13:302–308
Huang Y, Xiao B, Xiong L (2007) Characterization of a stress responsive proteinase inhibitor gene with positive effect in improving drought resistance in rice. Planta 226:73–85
Ingram J, Bartels D (1996) The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol 47:377–403
Koiwa H, Bressan RA, Hasegawa PM (1997) Regulation of protease inhibitors and plant defense. Trends Plant Sci 2:379–384
Koiwa H, Shade RE, Zhu-Salzman K, D’Urzo MP, Murdock LL, Bressan RA, Hasegawa PM (2000) A plant defensive cystatin (soyacystatin) targets cathepsin L-like digestive cysteine proteinases (DvCALs) in the larval midgut of western corn rootworm (Diabrotica virgifera virgifera). FEBS Lett 471:67–70
Koizumi M, Yamaguchi-Shinozaki K, Tsuji H, Shinozaki K (1993) Structures and expression of two genes that encode distinct drought-inducible cysteine proteinases in Arabidopsis thaliana. Gene 129:175–182
Kondo H, Abe K, Nishimutra I, Watanabe H, Emori Y, Arai S (1990) Two distinct cystatin species in rice seeds with different specificities against cysteine proteinases. Molecular cloning, expression, and biochemical studies on oryzacystatin-II. J Biol Chem 265:15832–15837
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lim CO, Lee SI, Chung WS, Park SH, Hwang I, Cho MJ (1996) Characterization of a cDNA encoding cysteine proteinase inhibitor from Chinese cabbage (Brassica campestris L. ssp. pekinensis) flower buds. Plant Mol Biol 30:373–379
Liu SK, Zhang XX (2004) Expression and purification of a novel rice (Oryza sativa L.) mitochondrial ATP synthase small subunit in Escherichia coli. Protein Expr Purif 37:306–310
Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1998) The transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10:1391–1406
Liu S, Cheng Y, Zhang X, Guan Q, Nishiuchi S, Hase K, Takano T (2007) Expression of an NADP-malic enzyme gene in rice (Oryza sativa. L) is induced by environmental stresses; over-expression of the gene in Arabidopsis confers salt and osmotic stress tolerance. Plant Mol Biol 64:49–58
Lu ZQ, Liu DL, Liu SK (2007) Two rice cytosolic ascorbate peroxidases differentially improve salt tolerance in transgenic Arabidopsis. Plant Cell Rep 26:1909–1917
Massonneau A, Condamine P, Wisniewski JP, Zivy M, Rogowsky PM (2005) Maize cystatins respond to developmental cues, cold stress and drought. Biochim Biophys Acta 1729:186–199
Menezes-Benavente L, Teixeira FK, Kamei CLA, Margis-Pinheiro M (2004) Salt stress induces altered expression of genes encoding antioxidant enzymes in seedlings of a Brazilian indica rice (Oryza sativa L.). Plant Sci 166:323–331
Mosolov VV, Valueva TA (2005) Proteinase inhibitors and their function in plants: a review. Appl Biochem Microb 41:227–246
Oppert B, Morgan TD, Hartzer K, Lenarcic B, Galesa K, Brzin J, Turk V, Yoza K, Ohtsubo K, Kramer KJ (2003) Effects of proteinase inhibitors on digestive proteinases and growth of the red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Comp Biochem Physiol 134:481–490
Pernas M, Sanchez-Monge R, Gomez L, Salcedo G (1998) A chestnut seed cystatin differentially effective against cysteine proteinases from closely related pests. Plant Mol Biol 38:1235–1242
Pernas M, Sanchez-Monge R, Gomez L, Salcedo G (2000) Biotic and abiotic stress can induce cystatin expression in chestnut. FEBS Lett 467:206–210
Sachs MM, Freeling M, Okimoto R (1980) The anaerobic proteins of maize. Cell 20:761–767
Sakuma Y, Maruyama K, Osakabe Y, Qin F, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2006) Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 18:1292–1309
Seki M, Narusaka M, Abe H, Kasuga M, Yamaguchi-Shinozaki K, Carninci P, Hayashizaki Y, Shinozaki K (2004) Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. Plant Cell 13:61–72
Shinozaki K, Yamaguchi-Shinozaki K (2000) Molecular responses to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. Curr Opin Plant Biol 3:217–223
Shyu DJH, Chyan CL, Tzen JTC, Chou WM (2004) Molecular cloning, expression and functional characterization of a cystatin from pineapple stem. Biosci Biotechnol Biochem 68:1681–1689
Soares-Costa A, Beltramini LM, Thiemann OH, Henrique-Silva F (2002) A sugarcane cystatin: recombinant expression, purification, and antifungal activity. Biochem Biophys Res Commun 296:1194–1199
Takahashi F, Maruyama K, Fujita Y, Umezawa T, Yoshida R, Shinozaki K, Yamaguchi-Shinozaki K (2006) ABA-dependent multisite phosphorylation regulates the activity of a transcription activator AREB1. Proc Natl Acad Sci USA 103:1988–1993
Thomashow MF (1999) Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. Annu Rev Plant Physiol Plant Mol Biol 50:571–599
Vyver CV, Schneidereit J, Driscoll S, Turner J, Kunert K, Foyer CH (2003) Oryzacystatin I expression in transformed tobacco produces a conditional growth phenotype and enhances chilling tolerance. Plant Biotech J 1:101–112
Waldron C, Wegrich LM, Merlo PA, Walsh TA (1993) Characterization of a genomic sequence coding for potato multicystatin, an eight-domain cysteine proteinase inhibitor. Plant Mol Biol 23:801–812
Wang WX, Vinocur B, Altman A (2003) Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218:1–14
Wiśniewski K, Zagdańska B (2001) Genotype-dependant proteolytic response of spring wheat to water deficiency. J Exp Bot 52:1455–1463
Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6:251–264
Yang AH, Yeh KW (2005) Molecular cloning, recombinant gene expression, and antifungal activity of cystatin from taro (Colocasia esculenta cv. Kaosiung no.1). Planta 221:493–501
Zimmermann HM, Hartmann K, Schreiber L, Steudle E (2000) Chemical composition of apoplastic transport barriers in relation to radical hydraulic conductivity of corn root (Zea mays L.). Planta 210:302–311
Acknowledgements
This work was supported by a Grant-in-aid for Scientific Research (18658001) and by a grant from Heiwa Nakajima Foundation to T.T. Additional support was provided by the Heilongjiang Provincial Program for Distinguished Young Scholars (JC200609) to S.L. We thank Dr. K. Yamaguchi-Shinozaki for valuable suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, X., Liu, S. & Takano, T. Two cysteine proteinase inhibitors from Arabidopsis thaliana, AtCYSa and AtCYSb, increasing the salt, drought, oxidation and cold tolerance. Plant Mol Biol 68, 131–143 (2008). https://doi.org/10.1007/s11103-008-9357-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-008-9357-x