Abstract
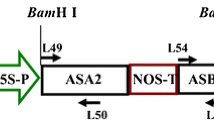
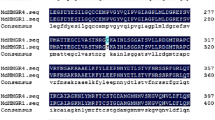
We fused four mutant omr1 alleles, encoding feedback-insensitive forms of Arabidopsis thaliana biosynthetic threonine dehydratase/deaminase (TD), to the CaMV 35S promoter and transformed these constructs into A. thaliana Columbia wild type plants. The mutant TD forms consisted of our previously isolated double mutant, omr1-1, and three new site-directed mutants, omr1-5, omr1-7, and omr1-8with single point mutations. We employed site-directed mutagenesis to assay the effects of amino acid substitutions in separate regulatory regions within the carboxy-terminal (C-term) allosteric end. TD assays and growth resistance to the isoleucine (Ile) toxic analog -O-methylthreonine (OMT) confirmed the desensitization to feedback inhibition and the viability of these mutant omr1 alleles as selectable markers, respectively. Two of the site-directed mutants, omr1-5 and omr1-7, appeared to influence one of the two separate Ile-binding sites and had a notable 13-fold and 15-fold increase in free Ile, respectively. The omr1-8 appeared to influence the other Ile-binding site and resulted in a 2-fold increase in free Ile. The transgenic omr1-1 double mutant affecting both Ile-binding sites, however, displayed a 106-fold increase in free Ile revealing a profound synergistic interplay between these separate Ile-binding sites. While all of the four omr1 alleles conferred resistance to elevated concentrations of OMT, the progeny of omr1-1 initial transformants exhibited a bushy phenotype at the rosette stage. On the other hand, progeny of transformants omr1-5, omr1-7, and omr1-8 had a normal phenotype, undistinguishable from wild type. Therefore, alleles omr1-5, omr1-7, and omr1-8, proved to be ideal as environmentally-friendly, dominant, selectable markers for plant transformation.
Similar content being viewed by others
References
Bechtold, N., Ellis, J. and Pelletier, G. 1993. In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. Paris Life Sci. 316: 1194–1199.
Bent, A.F., Kunkel, B.N., Dahlbeck, D., Brown, K.L., Schmidt, R., Giraudat, J., Leung, J. and Staskawicz, B.J. 1994. RPS2 of Arabidopsis thaliana: A leucine-rich repeat class of plant disease resistance genes. Science 265: 1856–1860.
Bevan, M. 1984. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 12: 8711–8721.
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the method of protein-dye binding. Anal. Biochem. 72: 248–254.
Brears, T., Liu, C., Knight, T.J. and Coruzzi G.M. 1993. Ectopic overexpression of asparagine synthetase in transgenic tobacco. Plant Physiol. 103: 1285–1290.
Bryan, J.K. (1990) Advances in the biochemistry of amino acid biosynthesis. In: B.J. Miflin, P.J. Lea (Eds.), The Biochemistry of Plants, Vol 16. Academic Press, New York, pp. 161–195.
Chinchilla, D., Schwarz, F.P. and Eisenstein, E. 1998. Amino acid substitutions in the C-terminal regulatory domain disrupt allosteric effector binding to biosynthetic threonine deaminase from Escherichia coli. J. Biol. Chem. 273: 23219–23224.
Dougall, D.K. 1970. Threonine deaminase from Paul's Scarlet Rose tissue cultures. Phytochemistry 9: 959–964.
Ebmeier, A., Allison, L., Cerutti, H. and Clemente, T. 2004. Evaluation pf the Escherichia coli threonine deaminase gene as a selectable marker for plant transformation. Planta 218: 751–758.
Eisenstein, E. 1991. Cloning, expression, purification, and characterization of biosynthetic threonine deaminase from Escherichia coli. J. Biol. Chem. 266: 5801–5807.
Fisher, K.E. and Eisenstein, E. 1993. An efficient approach to identify ilvA mutations reveals amino-terminal catalytic domain in biosynthetic threonine deaminase from Escherichia coli. J. Bacteriol. 175: 6605–6613.
Gallagher, D.T., Gilliland, G.L., Xiao, G., Zondlo, J., Fisher, K.E., Chinchilla, D. and Eisenstein, E. 1998. Structure and control of pyridoxal phosphate dependent allosteric threonine deaminase. Structure 6: 465–475.
Guex, N. and Peitsch, M.C. 1997. SWISS-MODEL and the Swiss-PdbViewer: An environment for comparative protein modelling. Electrophoresis 18: 2714–2723.
Guillouet, S., Rodal, A.A., An, G.-H., Lessard, P.A. and Sinskey, A.J. 1999. Expression of the Escherichia coli catabolic threonine dehydratase in Corynebacterium glutamicum and its effect on isoleucine production. Appl. Environ. Microbiol. 65: 3100–3107.
Halgand, F., Wessel, P.M., Laprevote, O. and Dumas, R. 2002. Biochemical and mass spectrometric evidence for quaternary structure modifications of plant threonine deaminase induced by isoleucine. Biochemistry 41: 13767–13773.
Hashiguchi, K., Kojima, H., Sato, K. and Sano, K. 1997. Effects of an Escherichia coli ilvA mutant gene encoding feedback-resistant threonine deaminase on L-isoleucine production by Brevibacterium flavum. Biosci. Biotechnol. Biochem. 61: 105–108.
Haughn, G.W. and Somerville, C. 1986. Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol. Gen. Genet. 204: 430–434.
Hildmann, T., Ebneth, M., Pena-Cortes, H., Sanchez-Serrano, J.J., Willmitzer, L. and Prat, S. 1992. General roles of abscisic and jasmonic acids in gene activation as a result of mechanical wounding. Plant Cell 4: 1157–1170.
Jefferson, R.A., Kavanagh, T.A. and Bevan, M.W. 1987. GUS fusions: ß-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6: 3901–3907.
John, S.J., Srivastava, V. and Guha-Mukherjee, S. 1995. Cloning and sequencing of chickpea cDNA coding for threonine deaminase. Plant Physiol. 107: 1023–1024.
Kielland-Brandt, M.C., Holmberg, S., Petersen, J.G.L. and Nilsson-Tillgren, T. 1984. Nucleotide sequence of the gene for threonine deaminase (ILV1) of Saccharomyces cerevisiae. Carlsberg Res. Commun. 49: 567–575.
Koncz, C. and Schell, J. 1986. The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol. Gen. Genet. 204: 383–396.
Lawther, R.P., Wek, R.C., Lopes, J.M., Pereira, R., Taillon, B.E. and Hatfield, G.W. 1987. The complete nucleotide sequence of the ilvGMEDA operon of Escherichia coli K-12. Nucl. Acids Res. 15: 2137–2155.
Monod, J., Wyman, J. and Changeux, J.-P. 1965. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 12: 88–118.
Mourad, G., Emerick, R., Marion, A. and Smith, A. 1998. Cloning and sequencing of a cDNA encoding threonine dehydratase/deaminase of Arabidopsis thaliana (Accession No. AF096281) (PGR98–199). Plant Physiol. 118: 1534.
Mourad, G., Emerick, R. and Smith, A. 2000. Molecular cloning and sequencing of a cDNA encoding an isoleucine feedback insensitive threonine dehydratase/deaminase of Arabidopsis thaliana line GM11b (Accession No. AF177212) (PGR00–020). Plant Physiol. 122: 619.
Mourad, G. and King, J. 1995. L-O-Methylthreonine-resistant mutant of Arabidopsis defective in isoleucine feedback regulation. Plant Physiol. 107: 43–52.
Mourad, G., Pandey, B. and King, J. 1993. Isolation and genetic analysis of a triazolopyrimidine-resistant mutant of Arabidopsis. J. Hered. 84: 91–96.
Nicholas, K.B. and Nicholas, H.B. 1997. GeneDoc: a tool for editing and annotating multiple sequence alignments. Distributed by the author. http:www.psc.edu/biomed/gendoc
Noctor, G., Arisi, A.-C.M., Jouanin, L. and Foyer, C.H. 1998. Manipulation of glutathione and amino acid biosynthesis in the chloroplast. Plant Physiol. 118: 471–482.
Peitsch, M.C. 1995. Protein modeling by E-mail. Bio Technol. 13: 658–660.
Peitsch, M.C. 1996. ProMod and Swiss-model: Internet-based tools for automated comparative protein modelling. Biochem. Soc. Trans. 24: 274–279.
Rhodes, D. and Wood, B. 2003. Amino acid and onium compound extraction. Department of Horticulture and Landscape Architecture, Purdue University. http://www. hort.purdue.edu/rhodcr/hort64oc/method:/me00001.htm.
Samach, A., Hareven, D., Gutfinger, T., Ken-Dror, S. and Lifschitz, E. 1991. Biosynthetic threonine deaminase gene of tomato: Isolation, structure, and upregulation in floral organs. Proc. Natl. Acad. Sci. USA 88: 2678–2682.
Schuller, D.J., Grant, G.A. and Banaszak, L.J. 1995. The allosteric ligand site in the Vmax-type cooperative enzyme phosphoglycerate dehydrogenase. Nat. Struct. Biol. 2: 69–76.
Sharma, R.K. and Mazumder, R. 1970. Purification, properties, and feedback control of L-threonine dehydratase from spinach. J. Biol. Chem. 245: 3008–3014.
Shaul, O. and Galili, G. 1992a. Increased lysine synthesis in tobacco plants that express high levels of bacterial dihydropicolinate synthase in their chloroplasts. Plant J. 2: 203–209.
Shaul, O. and Galili, G. 1992b. Threonine overproduction in transgenic tobacco plants expressing a mutant desensitized aspartate kinase of Escherichia coli. Plant Physiol. 100: 1157–1163.
Shintani, D. and DellaPenna, D. 1998. Elevating the Vitamin E content of plants through metabolic engineering. Science 282: 2098–2100.
Singh, B.K. and Shaner, D.L. 1995. Biosynthesis of branched chain amino acids: From test tube to field. Plant Cell 7: 935–944.
Slater, S., Mitsky, T.A., Houmiel, K.L., Hao, M., Reiser, S.E., Taylor, N.B., Tran, M., Valentin, H.E., Rodriguez, D.J., Stone, D.A., Padgette, S.R., Kishore, G. and Gruys, K.J. 1999. Metabolic engineering of Arabidopsis and Brassica for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production. Nat. Biotechnol. 17: 1011–1016.
Taillon, B.E., Little, R. and Lawther, R.P. 1988. Analysis of the functional domains of biosynthetic threonine deaminase by comparison of the amino acid sequences of three wild-type alleles to the amino acid sequence of biodegradative threonine deaminase. Gene 63: 245–252.
Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F. and Higgins, D.G. 1997. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucl. Acids Res. 25: 4876–4882.
Traber, M.G. and Sies, H. 1996. Vitamin E in humans: Demand and delivery. Annu. Rev. Nutr. 16: 321–347.
Umbarger, H.E. 1956. Evidence for a negative-feedback mechanism in the biosynthesis of isoleucine. Science 123: 848.
Umbarger, H.E. 1978. Amino acid biosynthesis and its regulation. Annu. Rev. Biochem. 47: 533–606.
Wessel, P.M., Graciet, E., Douce, R. and Dumas, R. 2000. Evidence for two distinct effector-binding sites in threonine deaminase by site-directed mutagenesis, kinetic, and binding experiments. Biochemistry 39: 15136–15143.
Zhu, X. and Galili, G. 2003. Increased lysine synthesis coupled with a knockout of its catabolism synergistically boosts lysine content and also transregulates the metabolism of other amino acids in Arabidopsis seeds. Plant Cell 15: 845–853.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Garcia, E.L., Mourad, G.S. A site-directed mutagenesis interrogation of the carboxy-terminal end of Arabidopsis thaliana threonine dehydratase/deaminase reveals a synergistic interaction between two effector-binding sites and contributes to the development of a novel selectable marker. Plant Mol Biol 55, 121–134 (2004). https://doi.org/10.1007/s11103-004-0500-z
Issue Date:
DOI: https://doi.org/10.1007/s11103-004-0500-z