Abstract
Introduction
Subcutaneous (SC) injectables have become more acceptable and feasible for administration of biologics and small molecules. However, efficient development of these products is limited to costly and time-consuming techniques, partially because absorption mechanisms and kinetics at the local site of injection remain poorly understood.
Objective
To bridge formulation critical quality attributes (CQA) of injectables with local physiological conditions to predict systemic exposure of these products.
Methodology
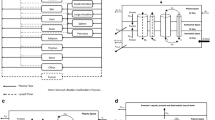
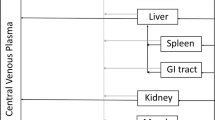
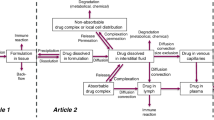
We have previously developed a multiscale, multiphysics computational model to simulate lymphatic absorption and whole-body pharmacokinetics of monoclonal antibodies. The same simulation framework was applied in this study to compute the capillary absorption of solubilized small molecule drugs that are injected subcutaneously. Sensitivity analyses were conducted to probe the impact by key simulation parameters on the local and systemic exposures.
Results
This framework was capable of determining which parameters had the biggest impact on small molecule absorption in the SC. Particularly, membrane permeability of a drug was found to have the biggest impact on drug absorption kinetics, followed by capillary density and drug diffusivity.
Conclusion
Our modelling framework proved feasible in predicting local transport and systemic absorption from the injection site of small molecules. Understanding the effect of these properties and how to model them may help to greatly expedite the development process.












Similar content being viewed by others
References
Turner MR, Balu-Iyer S, v. Challenges and Opportunities for the Subcutaneous Delivery of Therapeutic Proteins. J Pharm Sci. 2018;107:1247–60.
Brohem CA, Cardeal LBD, Tiago M, Soengas MS, Barros SBD, Maria-Engler SS. Artificial skin in perspective: concepts and applications. Pigment Cell Melanoma Res. 2011;24:35–50.
Viola M, Sequeira J, Seica R, Veiga F, Serra J, Santos AC, Ribiero A. Subcutaneous delivery of monoclonal antibodies: How do we get there? J Control Release. 2018;286:301–14.
Mehta CH, Narayan R, Nayak UY. Computational modeling for formulation design. Drug Discov Today. 2019;24:781–8.
Zhao L, Tsakalozou E. The utility of in silico PBPK absorption modeling and simulation as a tool to develop bio-predictive dissolution methods. 2017. https://www.pharmacy.umaryland.edu/media/SOP/wwwpharmacyumarylandedu/centers/cersievents/dissolution/day2_hopi-lin.pdf. Accessed 8 Oct 2022.
Loisios-Konstantinidis I, Cristofoletti R, Fotaki N, Turner DB, Dressman J. Establishing virtual bioequivalence and clinically relevant specifications using in vitro 2 biorelevant dissolution testing and physiologically-based population pharmacokinetic modeling. Case example: Naproxen 4 5 6. Eur J Pharm Sci. 2020;143:105170.
Doki K, Darwich AS, Patel N, Rostami-Hodjegan A. Virtual bioequivalence for achlorhydric subjects: The use of PBPK modelling to assess the formulation-dependent effect of achlorhydria. Eur J Pharm Sci. 2017;109:111–20.
Rostami A. Virtual Bioequivalence (VBE) requirements for prudent use of PBPK in uncharted territories. (n.d.). https://www.fda.gov/media/138036/download. Accessed 15 Nov 2022.
Zheng F, Hou P, Corpstein CD, Xing L, Li T. Multiphysics Modeling and Simulation of Subcutaneous Injection and Absorption of Biotherapeutics: Model Development. Pharm Res. 2021;38:607–24.
Zheng F, Hou P, Corpstein CD, Park K, Li T. Multiscale pharmacokinetic modeling of systemic exposure of subcutaneously injected biotherapeutics. J Control Release. 2021;337:407–16.
Hou P, Zheng F, Corpstein CD, Xing L, Li T. Multiphysics Modeling and Simulation of Subcutaneous Injection and Absorption of Biotherapeutics: Sensitivity Analysis. Pharm Res. 2021;38:1011–30.
Scallan J, Huxley VH, Korthuis RJ. Capillary Fluid Exchange: Regulation, Functions, and Pathology. Integ Syst Physiol: from Mole Func Dis. 2010;2:1–94.
Fleischer S, Tavakol DN, Vunjak-Novakovic G. From Arteries to Capillaries: Approaches to Engineering Human Vasculature. Adv Funct Mater. 2020;30:1910811.
Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran K, Straubhaar J, Nicoloro S, Czech M, Thompson M, Perugini R, Corvera S. Depot-Specific Differences and Insufficient Subcutaneous Adipose Tissue Angiogenesis in Human Obesity. Circulation. 2011;123:186–94.
Mathura RA, Russell-Puleri S, Cancel LM, Tarbell JM. Hydraulic conductivity of endothelial cell-initiated arterial cocultures. Ann Biomed Eng. 2014;42:763–75.
Noddeland H. Influence of body posture on transcapillary pressures in human subcutaneous tissue. Scand J Clin Lab Invest. 1982;42:131–8.
Ibrahim R, Nitsche JM, Kasting GB, Scallan J, Huxley VH, Korthuis RJ. Dermal clearance model for epidermal bioavailability calculations. J Pharm Sci. 2010;101:2094–108.
DeMaio L, Tarbell JM, Scaduto RC, Gardner TW, Antonetti DA. A transmural pressure gradient induces mechanical and biological adaptive responses in endothelial cells. Am J Physiol Heart Circ Physiol. 2004;286:H731–41.
Sill HW, Chang YS, Artman JR, Frangos JA, Hollis TM, Tarbell JM. Shear stress increases hydraulic conductivity of cultured endothelial monolayers. Am J Phys. 1995;268(2 Pt 2):H535–43.
Schmidt D, Lynch J. Evaluation of the reproducibility of Parallel Artificial Membrane Permeation Assays (PAMPA). (n.d.). https://www.sigmaaldrich.com/US/en/technical-documents/technical-article/research-and-disease-areas/pharmacology-and-drug-discovery-research/evaluation-of-the-reproducibility-of-pampa. Accessed 8 Sep 2021.
Fort JJ, Shao Z, Mitra AK. Transport and degradation characteristics of methotrexate dialkyl ester prodrugs across tape-stripped hairless mouse skin. Int J Pharm. 1993;100:233–9.
Massironi SMG, Giacóia MR, Maiorka PC, Kipnis TL, Dagli MLZ. Skin morphology of the mutant hairless USP mouse. Braz J Med Biol Res. 2005;38:33–9.
Pichlmeier U, Heuer K-U. Subcutaneous administration of methotrexate with a prefilled autoinjector pen results in a higher relative bioavailability compared with oral administration of methotrexate. Clin Exp Rheumatol. 2014;32:563–71.
Le Merdy M, Mullin J, Lukacova V. Development of PBPK model for intra-articular injection in human: methotrexate solution and rheumatoid arthritis case study. J Pharmacokinet Pharmacodyn. 2021;48(6):909–22.
Halpern V, Combes SL, Dorflinger LJ, Weiner DH, Archer DF. Pharmacokinetics of subcutaneous depot medroxyprogesterone acetate injected in the upper arm. Contraception. 2014;89:31–5.
Michel CC. Capillary permeability and how it may change. J Physiol. 1988;404:1–29.
Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Järvinen T, Jouko S. Prodrugs: design and clinical applications. Nat Rev Drug Discov. 2008;7:255–70.
Fiehn C. Methotrexate transport mechanisms: the basis for targeted drug delivery and ß-folate-receptor-specific treatment. Clin Exp Rheumatol. 2010;28:S40–5.
McCabe MC, Hill RC, Calderone K, Cui Y, Yan Y, Quan T, Fisher G, Hansen K. Alterations in extracellular matrix composition during aging and photoaging of the skin. Matrix Biol Plus. 2020;8:100041.
Chen W, Yung BC, Qian ZY, Chen XY. Improving long-term subcutaneous drug delivery by regulating material-bioenvironment interaction. Adv Drug Deliv Rev. 2018;127:20–34.
Beissner N, Albero AB, Fuller J, Kellner T, Lauterboeck L, Liang JH, Bol M, Glasmacher B, Muller-Goymann C, Reichl S. Improved in vitro models for preclinical drug and formulation screening focusing on 2D and 3D skin and cornea constructs. Eur J Pharm Biopharm. 2018;126:57–66.
Kinnunen HM, Sharma V, Contreras-Rojas LR, Yu Y, Alleman C, Sreedhara A, Fischer S, Khawli L, Yohe S, Bumbaca D, Patapoff T, Daughterty A, Mrsny R. A novel in vitro method to model the fate of subcutaneously administered biopharmaceuticals and associated formulation components. J Control Release. 2015;214:94–102.
Bennion BJ, Be NA, McNerney MW, Lao V, Carlson EM, Valdez CA, Malfatti MA, Enright HA, Hguyen TH, Lightstone FC, Carpenter TS. Predicting a Drug’s Membrane Permeability: A Computational Model Validated With in Vitro Permeability Assay Data. J Phys Chem. 2017;121(20):5228–37.
Acknowledgements
We thank Allen Chao Endowment for supporting this research work.
Author information
Authors and Affiliations
Contributions
CC drafted the manuscript, conducted simulations, and analyzed results. PH developed the simulation model. KP provided guidance of the research. TL directed the study and revised the manuscript.
Corresponding author
Ethics declarations
Conflict of Interests
None.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Corpstein, C.D., Hou, P., Park, K. et al. Multiphysics Simulation of Local Transport and Absorption Coupled with Pharmacokinetic Modeling of Systemic Exposure of Subcutaneously Injected Drug Solution. Pharm Res 40, 2873–2886 (2023). https://doi.org/10.1007/s11095-023-03546-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-023-03546-5