Abstract
Introduction
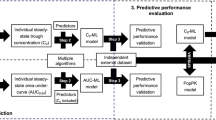
Vancomycin is one of the antibiotics most used in neonates. Continuous infusion has many advantages over intermittent infusions, but no consensus has been achieved regarding the optimal initial dose. The objectives of this study were: to develop a Machine learning (ML) algorithm based on pharmacokinetic profiles obtained by Monte Carlo simulations using a population pharmacokinetic model (POPPK) from the literature, in order to derive the best vancomycin initial dose in preterm and term neonates, and to compare ML performances with those of an literature equation (LE) derived from a POPPK previously published.
Materials and methods
The parameters of a previously published POPPK model of vancomycin in children and neonates were used in the mrgsolve R package to simulate 1900 PK profiles. ML algorithms were developed from these simulations using Xgboost, GLMNET and MARS in parallel, benchmarked and used to calculate the ML first dose. Performances were evaluated in a second simulation set and in an external set of 82 real patients and compared to those of a LE.
Results
The Xgboost algorithm yielded numerically best performances and target attainment rates: 46.9% in the second simulation set of 400–600 AUC/MIC ratio vs. 41.4% for the LE model (p = 0.0018); and 35.3% vs. 28% in real patients (p = 0.401), respectively). The Xgboost model resulted in less AUC/MIC > 600, thus decreasing the risk of nephrotoxicity.
Conclusion
The Xgboost algorithm developed to estimate the initial dose of vancomycin in term or preterm infants has better performances than a previous validated LE and should be evaluated prospectively.




Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Berlak N, Shany E, Ben-Shimol S, Chertok IA, Goldinger G, Greenberg D, et al. Late onset sepsis: comparison between coagulase-negative staphylococci and other bacteria in the neonatal intensive care unit. Infect Dis (Lond). 2018;50:764–70.
Pham JT. Challenges of Vancomycin Dosing and Therapeutic Monitoring in Neonates. The Journal of Pediatric Pharmacology and Therapeutics [Internet]. 2020 [cited 2021 Nov 24];25:476–84. Available from: https://meridian.allenpress.com/jppt/article/25/6/476/443970/Challenges-of-Vancomycin-Dosing-and-Therapeutic
Chung E, Sen J, Patel P, Seto W. Population Pharmacokinetic Models of Vancomycin in Paediatric Patients: A Systematic Review. Clin Pharmacokinet. 2021;60:985–1001.
Dao K, Guidi M, André P, Giannoni E, Basterrechea S, Zhao W, et al. Optimisation of vancomycin exposure in neonates based on the best level of evidence. Pharmacol Res. 2020;154: 104278.
Lestner JM, Hill LF, Heath PT, Sharland M. Vancomycin toxicity in neonates: a review of the evidence. Curr Opin Infect Dis. 2016;29:237–47.
Elyasi S, Khalili H, Dashti-Khavidaki S, Mohammadpour A. Vancomycin-induced nephrotoxicity: mechanism, incidence, risk factors and special populations. A literature review. Eur J Clin Pharmacol. 2012;68:1243–55.
Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: A revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77:835–64.
Round A, Clifton E, Stachow L, Mittal S, Yadav K, Ashraf H, et al. Continuous infusion of vancomycin improved therapeutic levels in term and preterm infants. J Perinatol. 2021;41:1459–66.
Gwee A, Cranswick N, Metz D, Coghlan B, Daley AJ, Bryant PA, et al. Neonatal vancomycin continuous infusion: still a confusion? Pediatr Infect Dis J. 2014;33:600–5.
Gwee A, Cranswick N, McMullan B, Perkins E, Bolisetty S, Gardiner K, et al. Continuous Versus Intermittent Vancomycin Infusions in Infants: A Randomized Controlled Trial. Pediatrics. 2019;143: e20182179.
Girand HL. Continuous Infusion Vancomycin in Pediatric Patients: A Critical Review of the Evidence. J Pediatr Pharmacol Ther. 2020;25:198–214.
Elmokadem A, Riggs MM, Baron KT. Quantitative Systems Pharmacology and Physiologically-Based Pharmacokinetic Modeling With mrgsolve: A Hands-On Tutorial. CPT Pharmacometrics Syst Pharmacol. 2019;8:883–93.
Woillard J-B, Labriffe M, Prémaud A, Marquet P. Estimation of drug exposure by machine learning based on simulations from published pharmacokinetic models: The example of tacrolimus. Pharmacol Res. 2021;167: 105578.
Jacqz-Aigrain E, Leroux S, Thomson AH, Allegaert K, Capparelli EV, Biran V, et al. Population pharmacokinetic meta-analysis of individual data to design the first randomized efficacy trial of vancomycin in neonates and young infants. J Antimicrob Chemother. 2019;74:2128–38.
Labriffe M, Woillard J-B, Debord J, Marquet P. Machine learning algorithms to estimate everolimus exposure trained on simulated and patient pharmacokinetic profiles. CPT Pharmacometrics Syst Pharmacol. 2022. https://doi.org/10.1002/psp4.12810.
Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59.
Vergnano S, Menson E, Kennea N, Embleton N, Russell AB, Watts T, et al. Neonatal infections in England: the NeonIN surveillance network. Arch Dis Child Fetal Neonatal Ed. 2011;96:F9-14.
Fenton TR, Nasser R, Eliasziw M, Kim JH, Bilan D, Sauve R. Validating the weight gain of preterm infants between the reference growth curve of the fetus and the term infant. BMC Pediatr. 2013;13:92.
Bateman DA, Thomas W, Parravicini E, Polesana E, Locatelli C, Lorenz JM. Serum creatinine concentration in very-low-birth-weight infants from birth to 34–36 wk postmenstrual age. Pediatr Res. 2015;77:696–702.
Zhao W, Lopez E, Biran V, Durrmeyer X, Fakhoury M, Jacqz-Aigrain E. Vancomycin continuous infusion in neonates: dosing optimisation and therapeutic drug monitoring. Arch Dis Child. 2013;98:449–53.
Kuhn M, Wickham H. tidymodels: Easily Install and Load the ‘Tidymodels’ Packages version 0.1.0 from CRAN. [Internet]. Available from: https://rdrr.io/cran/tidymodels/
Chen T, Guestrin C. XGBoost: A Scalable Tree Boosting System. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining [Internet]. San Francisco California USA: ACM; 2016 [cited 2021 Jun 30]. p. 785–94. Available from: https://doi.org/10.1145/2939672.2939785
Friedman JH, Roosen CB. An introduction to multivariate adaptive regression splines. Stat Methods Med Res. 1995;4:197–217.
Friedman J, Hastie T, Tibshirani R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw. 2010;33:1–22.
Leroux S, Jacqz-Aigrain E, Biran V, Lopez E, Madeleneau D, Wallon C, et al. Clinical Utility and Safety of a Model-Based Patient-Tailored Dose of Vancomycin in Neonates. Antimicrob Agents Chemother [Internet]. 2016 [cited 2021 Dec 13];60:2039–42. Available from: https://doi.org/10.1128/AAC.02214-15
Gomez L, Boegler D, Epiard C, Alin L, Arata-Bardet J, Caspar Y, et al. Implementation of a Vancomycin Dose-Optimization Protocol in Neonates: Impact on Vancomycin Exposure, Biological Parameters, and Clinical Outcomes. Antimicrob Agents Chemother. 2022;66: e0219121.
Jorgensen SCJ, Dersch-Mills D, Timberlake K, Stewart JJ, Gin A, Dresser LD, et al. AUCs and 123s: a critical appraisal of vancomycin therapeutic drug monitoring in paediatrics. J Antimicrob Chemother. 2021;76:2237–51.
Hornik CP, Fort P, Clark RH, Watt K, Benjamin DK, Smith PB, et al. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Human Development [Internet]. 2012 [cited 2022 Jan 4];88:S69–74. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378378212700191
Smits A, Kulo A, N de Hoon J, Allegaert K. Pharmacokinetics of Drugs in Neonates: Pattern Recognition Beyond Compound Specific Observations. CPD [Internet]. 2012 [cited 2022 Jan 4];18:3119–46. Available from: http://www.eurekaselect.com/openurl/content.php?genre=article&issn=1381-6128&volume=18&issue=21&spage=3119
Woillard J-B, Salmon Gandonnière C, Destere A, Ehrmann S, Merdji H, Mathonnet A, et al. A Machine Learning Approach to Estimate the Glomerular Filtration Rate in Intensive Care Unit Patients Based on Plasma Iohexol Concentrations and Covariates. Clin Pharmacokinet. 2021;60:223–33.
Acknowledgements
The authors acknowledge the kind contribution of the children and their parents
Author information
Authors and Affiliations
Contributions
LP & JBW: made substantial contributions to the conception, design of the work; acquisition, analysis, interpretation of data and drafted the work, PE : made substantial contributions to the acquisition and analysis, PM and ML made substantial contributions to the interpretation of data and EJA made substantial contributions to the acquisition, interpretation of data and draft. AD built the shiny application.
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no competing interests to declare in relation to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ponthier, L., Ensuque, P., Destere, A. et al. Optimization of Vancomycin Initial Dose in Term and Preterm Neonates by Machine Learning. Pharm Res 39, 2497–2506 (2022). https://doi.org/10.1007/s11095-022-03351-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03351-6