Abstract
Purpose
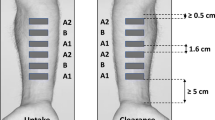
Skin sampling by tape stripping measures the local bioavailability of topical drug products in the stratum corneum (SC). The goal of the current study was to evaluate the impact of different investigators in studies that utilize a tape stripping protocol designed to minimize investigator variability.
Methods
Two open-label clinical studies compared two lidocaine patches and a diclofenac patch and solution in twelve healthy volunteers. The mass of drug was determined in SC samples collected on tape strips at three time points following product removal in duplicate by two investigators. Investigator results were compared with each other and with results for the diclofenac solution measured by another laboratory using a similar protocol.
Results
For drug mass, the geometric mean ratio comparing two investigators is within the acceptable bioequivalence interval for most measurement times and drug products. Drug uptake into the SC from the diclofenac solution was not statistically different from that determined in another laboratory. The average flux from the SC over the clearance intervals for the four drug products correspond well with flux measurements from in vitro permeation tests.
Conclusions
Results from different investigators are reproducible within the limitations of measurement variability, which can be managed by increasing volunteer numbers.











Similar content being viewed by others
References
United States Food and Drug Administration. Approved drug products with therapeutic equivalence evaluations (orange book). 2021. [Internet]. [cited 2021 Sep 27]. Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/approved-drug-products-therapeutic-equivalence-evaluations-orange-book. Accessed 27 Sept 2021
Midha KK, McKay G. Bioequivalence; its history, practice, and future. AAPS J [Internet]. 2009 Dec;11(4):664–70. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19806461
Petersen B, Rovati S. Diclofenac epolamine (Flector) patch: evidence for topical activity. Clin Drug Investig [Internet]. 2009;29(1):1–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19067470
Herkenne C, Alberti I, Naik A, Kalia YN, Mathy F-X, Préat V, et al. In vivo methods for the assessment of topical drug bioavailability. Pharm Res [Internet]. 2008 Jan;25(1):87–103. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17985216
Chang R-K, Raw A, Lionberger R, Yu L. Generic development of topical dermatologic products: formulation development, process development, and testing of topical dermatologic products. AAPS J [Internet]. 2013 Jan;15(1):41–52. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23054971
Miranda M, Cardoso C, Vitorino C. Quality and equivalence of topical products: a critical appraisal. Eur J Pharm Sci [Internet]. 2019 Oct 15;105082. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31626969
United States Food and Drug Administration. Draft guidance on Lidocaine. 2018. [Internet]. [cited 2020 Feb 13]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Lidocaine_draft_Topical.patch_RLD020612_RC10-18.pdf . Accessed 13 Feb 2020
United States Food and Drug Administration. Draft guidance on acyclovir. 2016. https://www.accessdata.fda.gov/drugsatfda_docs/psg/Acyclovir_topical%20cream_RLD%2021478_RV12-16.pdf. Accessed 13 Feb 2020.
Dandamudi S. In vitro bioequivalence data for a topical product: bioequivalence review perspective. Oral presentation at: FDA Public Workshop Topical Dermatological Generic Drug Products; October 20, 2017 [Internet]. [cited 2020 Feb 13]. Available from: https://www.fda.gov/media/110389/download. Accessed 13 Feb 2020
United States Food and Drug Administration. Draft guidance on Ivermectin. 2019. [Internet]. [cited 2021 Apr 27]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Ivermectin.TopicalCream1NDA206255PSGPageRVMay2019.pdf. Accessed 27 April 2021
United States Food and Drug Administration. Draft guidance on Clindamycin Phosphate. 2019. [Internet]. [cited 2021 Apr 27]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_050615.pdf. Accessed 27 April 2021
Shah VP, Yacobi A, Rădulescu FŞ, Miron DS, Lane ME. A science based approach to topical drug classification system (TCS). Int J Pharm [Internet]. 2015 Aug;491(1–2):21–5. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378517315005220
Advances in Topical Bioequivalence Assessments: Characterization-Based Approaches. Oral Presentation at University of Maryland Center of Excellence in Regulatory Science and Innovation (M-CERSI) [Internet]. [cited 2021 Apr 27]. Available from: https://cersi.umd.edu/sites/cersi.umd.edu/files/Day.1-2SamRaneyUofM-CERSI2020_0.pdf. Accessed 27 April 2021
United States Food and Drug Administration. Guidance for industry: Statistical Approaches to Establishing Bioequivalence. 2001. [Internet]. [cited 2021 Sep 27]. Available from: https://www.fda.gov/downloads/drugs/guidances/ucm070244.pdf. Accessed 13 Feb 2020
N’Dri-Stempfer B, Navidi WC, Guy RH, Bunge AL. Improved bioequivalence assessment of topical dermatological drug products using dermatopharmacokinetics. Pharm Res [Internet]. 2009 Feb;26(2):316–28. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18941872
Cordery SF, Pensado A, Chiu WS, Shehab MZ, Bunge AL, Delgado-Charro MB, et al. Topical bioavailability of diclofenac from locally-acting, dermatological formulations. Int J Pharm [Internet]. 2017 Aug 30;529(1–2):55–64. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28636892
Pensado A, Chiu WS, Cordery SF, Rantou E, Bunge AL, Delgado-Charro MB, et al. Stratum corneum sampling to assess bioequivalence between topical acyclovir products. Pharm Res [Internet]. 2019 Nov 14;36(12):180. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31728737
Escobar-Chávez JJ, Merino-Sanjuán V, López-Cervantes M, Urban-Morlan Z, Piñón-Segundo E, Quintanar-Guerrero D, et al. The tape-stripping technique as a method for drug quantification in skin. J Pharm Pharm Sci [Internet]. 2008;11(1):104–30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18445368
European Medicines Agency. Draft guideline on quality and equivalence of topical products [Internet]. [cited 2021 Apr 27]. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/draft-guideline-quality-equivalence-topical-products_en.pdf. Accessed 27 April 2021
Yacobi A, Shah VP, Bashaw ED, Benfeldt E, Davit B, Ganes D, et al. Current challenges in bioequivalence, quality, and novel assessment technologies for topical products. Pharm Res [Internet]. 2014 Apr;31(4):837–46. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24395404
Davies NM, Anderson KE. Clinical pharmacokinetics of diclofenac. Therapeutic insights and pitfalls. Clin Pharmacokinet [Internet]. 1997 Sep;33(3):184–213. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9314611
United States Food and Drug Administration. Draft guidance for industry: topical dermatological drug product NDAs and ANDAs—in vivo bioavailability, bioequivalence, in vitro release, and associated studies. 1998. [Internet]. [cited 2020 Mar 6]. Available from: https://pdfs.semanticscholar.org/18c4/a3a9696003e353f2cc7830560e828a89eed5.pdf?_ga=2.61590767.2143012472.1583526789-1250934800.1583526789. Accessed 06 March 2020
Shah VP, Flynn GL, Yacobi A, Maibach HI, Bon C, Fleischer NM, et al. Bioequivalence of topical dermatological dosage forms--methods of evaluation of bioequivalence. AAPS/FDA Workshop on “Bioequivalence of Topical Dermatological Dosage Forms-- Methods of Evaluating Bioequivalence”, September 4–6, 1996, Bethesda, Md. Skin Pharmacol Appl Skin Physiol [Internet]. 11(2):117–24. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9603663. Accessed 22 Jan 2020
Draft guidance for industry on topical dermatological drug product NDAs and ANDAs-in vivo bioavailability, bioequivalence, in vitro release and associated studies; withdrawal (2002). Fed Reg. 2002;67:35122–3. https://www.federalregister.gov/articles/2002. Accessed 13 Feb 2020
Raney SG, Franz TJ, Lehman PA, Lionberger R, Chen M-L. Pharmacokinetics-based approaches for bioequivalence evaluation of topical dermatological drug products. Clin Pharmacokinet [Internet]. 2015 Nov;54(11):1095–106. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26063051
N’Dri-Stempfer B, Navidi WC, Guy RH, Bunge AL. Optimizing metrics for the assessment of bioequivalence between topical drug products. Pharm Res [Internet]. 2008 Jul;25(7):1621–30. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18449629
Navidi W, Hutchinson A, N’Dri-Stempfer B, Bunge A. Determining bioequivalence of topical dermatological drug products by tape-stripping. J Pharmacokinet Pharmacodyn [Internet]. 2008 Jun;35(3):337–48. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18465213
Shukla S. Evaluation of skin tape stripping in healthy human volunteers as a methodology for quantifying local drug bioavailability from dermal products (Document No. 27993494) [Doctoral dissertation, University of Maryland School of Pharmacy]. ProQuest Dissertations Publishing. 2020
M. Alice Maciel Tabosa, Sarah F. Cordery, K.A. Jane White, Annette L. Bunge, Richard H. Guy, M. Begona Delgado-Charro, “Skin pharmacokinetics of diclofenac and co-delivered excipients”. Intl J Pharm. 2022;614:121469. https://doi.org/10.1016/j.ijpharm.2022.121469
Lidoderm [package insert]. Chadds Ford, PA: Endo Pharmaceuticals Inc.; 2004. [Internet]. [cited 2020 Feb 13]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020612s014lbl.pdf. Accessed 13 Feb 2020
Lidocaine patch 5% [package insert].Morgantown, WV: Mylan Pharmaceuticals Inc.;2018. [Internet]. [cited 2020 Feb 13]. Available from: https://dailymed.nlm.nih.gov/dailymed/getFile.cfm?setid=5c66f3b9-6e04-47ab-8d94-21e89ceec154&type=pdf&name=5c66f3b9-6e04-47ab-8d94-21e89ceec154. Accesed. 13 Feb 2020
Drago S, Imboden R, Schlatter P, Buylaert M, Krähenbühl S, Drewe J. Pharmacokinetics of transdermal etofenamate and diclofenac in healthy volunteers. Basic Clin Pharmacol Toxicol [Internet]. 2017 Nov;121(5):423–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28561421
Acknowledgements
This work is supported in part by the University of Maryland Baltimore, School of Pharmacy Mass Spectrometry Center (SOP1841-IQB2014). We thank Drs. Priyanka Ghosh and Sam Raney at the United States Food and Drug Administration for their input into the current study. The views expressed in this manuscript do not reflect the official policies of the Department of Health and Human Services; nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
Funding
This publication was supported by the Food and Drug Administration (FDA) of the U.S. Department of Health and Human Services (HHS) as part of a financial assistance award [FAIN] (Research Award U01FD004947) totaling $2,350,000 with 100 percent funded by FDA]/HHS. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by FDA/HHS, or the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest statement
The authors have no financial or proprietary interests in any material discussed in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM1
(PDF 644 KB)
Rights and permissions
About this article
Cite this article
Shukla, S., Bunge, A.L., Hassan, H.E. et al. Investigator Impact on Reproducibility of Drug Bioavailability in Stratum Corneum Sampling by Tape Stripping. Pharm Res 39, 703–719 (2022). https://doi.org/10.1007/s11095-022-03199-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-022-03199-w