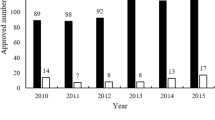
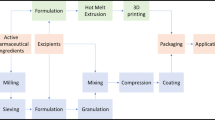
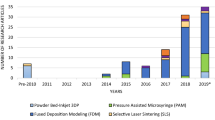
Abstract
Fixed-dose combination (FDC) products containing at least two different active pharmaceutical ingredients are designed to treat more effectively different pathologies as they have demonstrated to enhance patient compliance. However, the combination of multiple drugs within the same dosage form can bring many physicochemical and pharmacodynamic interactions. The manufacturing process of FDC products can be challenging, especially when it is required to achieve different drug release profiles within the same dosage form to overcome physicochemical drug interactions. Monolithic, multiple-layer, and multiparticulate systems are the most common type of FDCs. Currently, the main manufacturing techniques utilized in industrial pharmaceutical companies rely on the use of combined wet and dry granulation, hot-melt extrusion coupled with spray coating, and compression of bilayered tablets. Nowadays, personalized medicines are gaining importance in clinical settings and 3D printing is taking a highlighted role in the manufacturing of complex and personalized 3D solid dosage forms that could not be manufactured using conventional techniques. In this review, it will be discussed in detail current marketed FDC products and their application in several diseases with an especial focus on antimicrobial drugs. Current industrial conventional techniques will be compared with 3D printing manufacturing of FDCs.

Graphical Abstract








Similar content being viewed by others
References
Collier R. Reducing the "pill burden". Can Med Assoc J. 2012;184:E117–8.
Gautamand CS, Saha L. Fixed dose drug combinations (FDCs): rational or irrational: a view point. Br J Clin Pharmacol. 2008;65:795–6.
Bourliereand M, Pietri O. Hepatitis C virus therapy: no one will be left behind. Int J Antimicrob Agents. 2019;53:755–60.
AEMPS. Caduet, data sheet. https://cima.aemps.es/cima/dochtml/ft/67326/FT_67326.html (accessed 4th November 2019.
Lung T, Jan T, de Silva HA, Guggilla R, Maulik PK, Naik N. Fixed-combination, low-dose, triple-pill antihypertensive medication versus usual care in patients with mild-to-moderate hypertension in Sri Lanka: a within-trial and modelled economic evaluation of the TRIUMPH trial. Lancet. 2019;7:1359–66.
Farrell B, Shamji S, Monahan A, French Merkley V. Reducing polypharmacy in the elderly: cases to help you "rock the boat". Can Pharm J (Ott). 2013;146:243–4.
Schlosser R. Fixed-dose and fixed-ratio combination therapies in type 2 diabetes. Can J Diabetes. 2019;43:440–4.
Moriarty F, Bennett K, Fahey T. Fixed-dose combination antihypertensives and risk of medication errors. Heart. 2019;105:204–9.
Sadia M, Isreb A, Abbadi I, Isreb M, Aziz D, Selo A, et al. From 'fixed dose combinations' to 'a dynamic dose combiner': 3D printed bi-layer antihypertensive tablets. Eur J Pharm Sci. 2018;123:484–94.
List of medicine price. Retrieved the 8 May 2020 from: www.vademecum.es.
Hao J, Rodriguez-Monguio R, Seoane-Vazquez E. Fixed-dose combination drug approvals, patents and market exclusivities compared to single active ingredient pharmaceuticals. PLoS One. 2015;10:e0140708.
Khouri AS, Realini T, Fechtner RD. Fixed-Combination Drugs. In: Netland PA, editor. Glaucoma Medical Therapy. New York: Oxford University Press; 2008. p. 139–49.
Aronson JK. Medication errors resulting from the confusion of drug names. Expert Opin Drug Saf. 2004;3:167–72.
FDA. Drugs@FDA Approved Drug Products: estradiol cypionate; testosterone cypionate. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=017968 (accessed 27th September 2019.
Hao J, Rodriguez-Monguio R, Seoane-Vazquez E. Fixed-dose combination and single active ingredient drugs: a comparative cost analysis. Expert Rev Pharm Out. 2016;16:127–34.
NIH. Fixed-Dose Combination. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/results?cond=fixed-dose+combination&term=&cntry=&state=&city=&dist= (accessed 23rd March 2020.
Mirbagheri SA, Hasibi M, Abouzari M, Rashidi A. Triple, standard quadruple and ampicillin-sulbactam-based quadruple therapies for H. pylori eradication: a comparative three-armed randomized clinical trial. World J Gastroenterol. 2006;12:4888–91.
AEMPS. Septrin Forte, data sheet. https://cima.aemps.es/cima/dochtml/ft/58501/FT_58501.html (accessed 2nd December 2019.
Salazar-Austin N, Ordonez AA, Hsu AJ, Benson JE, Mahesh M, Menachery E, et al. Extensively drug-resistant tuberculosis in a young child after travel to India. Lancet Infect Dis. 2015;15:1485–91.
Blomberg B, Spinaci S, Fourie B, Laing R. The rationale for recommending fixed-dose combination tablets for treatment of tuberculosis. Bull World Health Organ. 2001;79:61–8.
Teixeira KSS, da Cruz Fonseca SG, de Moura LCB, de Moura MLR, Borges MHP, Barbosa EG, et al. Use of chemometrics to compare NIR and HPLC for the simultaneous determination of drug levels in fixed-dose combination tablets employed in tuberculosis treatment. J Pharm Biomed Anal. 2018;149:557–63.
New, Once-a-Day Fixed-Dose Combination Against Malaria Now Available. Retrieved the 8 May 2020 from: https://www.dndi.org/2007/media-centre/press-releases/new-once-a-day-fixed-dose-combination-against-malaria-now-available/.
Assi SB, Aba YT, Yavo JC, Nguessan AF, Tchiekoi NB, San KM, et al. Safety of a fixed-dose combination of artesunate and amodiaquine for the treatment of uncomplicated plasmodium falciparum malaria in real-life conditions of use in cote d'Ivoire. Malar J. 2017;16:8.
Mooreand RD, Chaisson RE. Natural history of HIV infection in the era of combination antiretroviral therapy. Aids. 1999;13:1933–42.
Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. New Engl J Med. 1997;337:734–9.
AEMPS. Combivir, data sheet. https://cima.aemps.es/cima/dochtml/ft/98058001/FT_98058001.html (accessed 2nd December 2019.
Siyawamwaya M, du Toit LC, Kumar P, Choonara YE, Kondiah P, Pillay V. 3D printed, controlled release, tritherapeutic tablet matrix for advanced anti-HIV-1 drug delivery. Eur J Pharm Biopharm. 2019;138:99–110.
AEMPS. Trizivir, data sheet. https://cima.aemps.es/cima/dochtml/ft/100156004/FT_100156004.html (accessed 4th May 2020.
AEMPS. Kivexa, data sheet. https://cima.aemps.es/cima/dochtml/ft/04298002/FT_04298002.html (accessed 4th May 2020.
AEMPS. Truvada, data sheet. https://cima.aemps.es/cima/dochtml/ft/04305001/FT_04305001.html 4th May 2020).
AEMPS. Atripla, data sheet. https://cima.aemps.es/cima/dochtml/ft/07430001/FT_07430001.html (accessed 4th May 2020.
AEMPS. Eviplera, data sheet. https://cima.aemps.es/cima/pdfs/ft/11737001/FT_11737001.pdf (accessed 4th May 2020.
AEMPS. Stribild, data sheet. https://cima.aemps.es/cima/dochtml/ft/113830001/FT_113830001.html (accessed 4th May 2020.
AEMPS. Triumeq, data sheet. https://cima.aemps.es/cima/dochtml/ft/114940001/FT_114940001.html (accessed 4th May 2020.
FDA. Dutrebis, data sheet. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=206510 (accessed 4th May 2020.
AEMPS. Genvoya, data sheet. https://cima.aemps.es/cima/pdfs/ft/1151061001/FT_1151061001.pdf (accessed 4th May 2020.
AEMPS. Odefsey, data sheet. https://cima.aemps.es/cima/pdfs/ft/1161112001/FT_1161112001.pdf (accessed 4th May 2020.
AEMPS. Descovy, data sheet. https://cima.aemps.es/cima/pdfs/ft/1161099003/FT_1161099003.pdf (accessed 4th May 2020.
FDA. Symfi, data sheet. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=022142 (accessed 4th May 2020.
AEMPS. Biktarvy, data sheet. https://cima.aemps.es/cima/pdfs/ft/1181289001/FT_1181289001.pdf (accessed 4th May 2020.
FDA. Cimduo, data sheet. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=022141 (accessed 4th May 2020.
AEMPS. Symtuza, data sheet. https://cima.aemps.es/cima/pdfs/ft/1171225001/FT_1171225001.pdf (accessed 4th May 2020.
FDA. Delstrigo, data sheet. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=210807 (accessed 4th May 2020.
FDA. Dovato, data sheet. https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=211994 (accessed 4th May 2020.
AEMPS. Juluca, data sheet. https://cima.aemps.es/cima/dochtml/ft/1181282001/FT_1181282001.html (accessed 4th May 2020.
AEMPS. Kaletra, data sheet. https://cima.aemps.es/cima/dochtml/ft/01172006/FT_01172006.html (accessed 2nd December 2019.
AEMPS. Evotaz, data sheet. https://cima.aemps.es/cima/pdfs/ft/1151025001/FT_1151025001.pdf (accessed 2nd December 2019.
AEMPS. Rezolsta, data sheet. https://cima.aemps.es/cima/pdfs/ft/114967001/FT_114967001.pdf (accessed 2nd December 2019.
Aidsmap. Types of antiretroviral medications. http://www.aidsmap.com/about-hiv/types-antiretroviral-medications (accessed 2nd December 2019.
Biswas P, Tambussi G, Lazzarin A. Access denied? The status of co-receptor inhibition to counter HIV entry. Expert Opin Pharmacother. 2007;8:923–33.
Rongand L, Perelson AS. Treatment of hepatitis C virus infection with interferon and small molecule direct antivirals: viral kinetics and modeling. Crit Rev Immunol. 2010;30:131–48.
Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006;55:1350–9.
Afdhal N, Zeuzem S, Kwo P, Chojkier M, Gitlin N, Puoti M, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370:1889–98.
AEMPS. Harvoni, data sheet. https://cima.aemps.es/cima/pdfs/ft/114958001/FT_114958001.pdf (accessed 5th October 2019.
Marksand K, Naggie S. Management of Hepatitis C in 2019. JAMA. 2019;322:355–6.
AEMPS. Zepatier, data sheet. https://cima.aemps.es/cima/dochtml/ft/1161119001/FT_1161119001.html (accessed 5th October 2019.
AEMPS. Maviret, data sheet. https://cima.aemps.es/cima/dochtml/ft/1171213001/FT_1171213001.html.
AEMPS. Epclusa, data sheet. https://cima.aemps.es/cima/dochtml/ft/1161116001/FT_1161116001.html (accessed 5th October 2019.
AEMPS. Vosevi, data sheet. https://cima.aemps.es/cima/dochtml/ft/1171223001/FT_1171223001.html (accessed 5th October 2019.
Junfeng M, Lovell MR, Mickle MH. Formulation and processing of novel conductive solution inks in continuous inkjet printing of 3-D electric circuits. IEEE Trans Electron Packag Manuf. 2005;28:265–73.
Shang D, Liu Y, Jiang F, Ji F, Wang H, Han X. Synergistic Antibacterial Activity of Designed Trp-Containing Antibacterial Peptides in Combination With Antibiotics Against Multidrug-Resistant Staphylococcus epidermidis. Front Microbiol. 2019;10:2719.
Gunnison JB, Kunishige E, Coleman VR, Jawetz E. The mode of action of Antibiotic synergism and antagonism: the effect in vitro on Bacteria not actively multiplying. Microbiology. 1955;13:509–18.
Vardakas KZ, Athanassaki F, Pitiriga V, Falagas ME. Clinical relevance of in vitro synergistic activity of antibiotics for multidrug-resistant gram-negative infections: a systematic review. J Global Antimicrob Resist. 2019;17:250–9.
Xu X, Xu L, Yuan G, Wang Y, Qu Y, Zhou M. Synergistic combination of two antimicrobial agents closing each other’s mutant selection windows to prevent antimicrobial resistance. Sci Rep. 2018;8:7237.
Desai D, Wang J, Wen H, Li X, Timmins P. Formulation design, challenges, and development considerations for fixed dose combination (FDC) of oral solid dosage forms. Pharm Dev Technol. 2013;18:1265–76.
Mandal U, Kumar-Pal T. Formulation and In Vitro Studies of a Fixed-Dose Combination of a Bilayer Matrix Tablet Containing Metformin HCl as Sustained Release and Glipizide as Immediate Release. Drug Dev Ind Pharm. 2008;34:305–13.
EMA. EMA Scientific Discussion on Atripla. https://www.ema.europa.eu/en/documents/scientific-discussion/atripla-epar-scientific-discussion_en.pdf (accessed 23rd March 2020.
EMA. EMA Assesment report on efavirez/emtricitabine/tenofovir disiproxil Mylan. https://www.ema.europa.eu/en/documents/assessment-report/efavirenz/emtricitabine/tenofovir-disoproxil-mylan-epar-public-assessment-report_en.pdf (accessed 23rd March 2020.
Caldwelland W, Kaushal M. Multiparticulate Technologies for Fixed-Dose Combinations. 2017, pp. 155–168.
Serrano DR, Walsh D, O'Connell P, Mugheirbi NA, Worku ZA, Bolas-Fernandez F, et al. Optimising the in vitro and in vivo performance of oral cocrystal formulations via spray coating. Eur J Pharm Biopharm. 2018;124:13–27.
Ziaee A, Albadarin AB, Padrela L, Femmer T, O'Reilly E, Walker G. Spray drying of pharmaceuticals and biopharmaceuticals: critical parameters and experimental process optimization approaches. Eur J Pharm Sci. 2019;127:300–18.
Ziaee A, O'Dea S, Howard-Hildige A, Padrela L, Potter C, Iqbal J, et al. Amorphous solid dispersion of ibuprofen: A comparative study on the effect of solution based techniques. Int J Pharm. 2019;572:118816.
Krause J, Breitkreutz J. Improving Drug Delivery in Paediatric Medicine. Pharm Med. 2008;22:41–50.
Desai U, Chaudhari P, Bhavsar D. Melt granulation: an alternative to traditional granulation techniques. Indian Drugs. 2013;50:5–13.
Vynckier AK, Dierickx L, Saerens L, Voorspoels J, Gonnissen Y, De Beer T, et al. Hot-melt co-extrusion for the production of fixed-dose combination products with a controlled release ethylcellulose matrix core. Int J Pharm. 2014;464:65–74.
Vynckier A-K, Dierickx L, Voorspoels J, Gonnissen Y, Remon JP, Vervaet C. Hot-melt co-extrusion: requirements, challenges and opportunities for pharmaceutical applications. J Pharm Pharmacol. 2014;66:167–79.
Walsh D, Serrano DR, Worku ZA, Norris BA, Healy AM. Production of cocrystals in an excipient matrix by spray drying. Int J Pharm. 2018;536:467–77.
Gascon N, Almansa C, Merlos M, Miguel Vela J, Encina G, Morte A, et al. Co-crystal of tramadol-celecoxib: preclinical and clinical evaluation of a novel analgesic. Expert Opin Investig Drugs. 2019;28:399–409.
Plata-Salamánand C, Tesson N. Co-crystals of tramadol and coxibs. In S.A. Laboratorios del Dr. Esteve (ed.), Vol. US 8.598,152 B2, United States, 2010.
NIH. Co-crystal E-58425 vs Tramadol and Celecoxib for Moderate to Severe Acute Pain After Bunionectomy. Phase III Clinical Trial. https://clinicaltrials.gov/ct2/show/NCT03108482 (accessed 26th March 2020.
Mohammed GA, Puri V, Bansal AK. Coprocessing of Nevirapine and Stavudine by spray drying. Pharm Dev Technol. 2008;13:299–310.
Battini S, Mannava MKC, Nangia A. Improved stability of tuberculosis drug fixed-dose combination using isoniazid-Caffeic acid and Vanillic acid Cocrystal. J Pharm Sci-Us. 2018;107:1667–79.
Swapna B, Maddileti D, Nangia A. Cocrystals of the tuberculosis drug isoniazid: polymorphism, Isostructurality, and stability. Cryst Growth Des. 2014;14:5991–6005.
Goheland MC, Jogani PD. A review of co-processed directly compressible excipients. J Pharm Pharm Sci. 2005;8:76–93.
Thakuriaand R, Sarma B. Drug-drug and drug-Nutraceutical Cocrystal/salt as alternative medicine for combination therapy: a crystal engineering approach. Crystals. 2018;8:101.
Smith L, Serrano DR, Mauger M, Bolas-Fernandez F, Dea-Ayuela MA, Lalatsa A. Orally bioavailable and effective Buparvaquone lipid-based Nanomedicines for visceral Leishmaniasis. Mol Pharm. 2018;15:2570–83.
Mendes C, Valentini G, Chamorro AF, Pinto JMO, Silva MAS, Parize AL. Supersaturating drug delivery system of fixed drug combination: sulfamethoxazole and trimethoprim. Expert Rev Anti-Infect Ther. 2019;17:841–50.
Raventós M, Duarte S, Alarcón R. Application and possibilities of supercritical CO2 extraction in food processing industry: an overview. Food Sci Technol Int. 2002;8:269–84.
Kelleher JF, Gilvary GC, Madi AM, Jones DS, Li S, Tian Y, et al. A comparative study between hot-melt extrusion and spray-drying for the manufacture of anti-hypertension compatible monolithic fixed-dose combination products. Int J Pharm. 2018;545:183–96.
Walsh D, Serrano DR, Worku ZA, Madi AM, O'Connell P, Twamley B, et al. Engineering of pharmaceutical cocrystals in an excipient matrix: spray drying versus hot melt extrusion. Int J Pharm. 2018;551:241–56.
Abebe A, Akseli I, Sprockel O, Kottala N, Cuitiño AM. Review of bilayer tablet technology. Int J Pharm. 2014;461:549–58.
Sadekand H, Dietel GL. Film-enrobed unitary-core medicament and the like. In B.G.P. Corp. (ed.), Vol. WO 91/04017, United States, 1990.
Clarkeand A, Doughty D. Development of Liquid Dispensing Technology for the Manufacture of Low Dose Drug Products. Continuous Manufacturing of Pharmaceuticals 2017, pp. 551–575.
Goyanes A, Scarpa M, Kamlow M, Gaisford S, Basit AW, Orlu M. Patient acceptability of 3D printed medicines. Int J Pharm. 2017;530:71–8.
Goyanes A, Madla CM, Umerji A, Duran Pineiro G, Giraldez Montero JM, Lamas Diaz MJ, et al. Automated therapy preparation of isoleucine formulations using 3D printing for the treatment of MSUD: first single-Centre, prospective, crossover study in patients. Int J Pharm. 2019;567:118497.
Lalatsa A, Fernández-García R, Serrano D. Market demands in 3D printing of pharmaceutical products. 2019, pp. 165–183.
Alhnan MA, Okwuosa TC, Sadia M, Wan K-W, Ahmed W, Arafat B. Emergence of 3D printed dosage forms: opportunities and challenges. Pharm Res. 2016;33:1817–32.
Haas R, Lohse S, Düllmann CE, Eberhardt K, Mokry C, Runke J. Development and characterization of a Drop-on-Demand inkjet printing system for nuclear target fabrication. Nucl Inst Methods Phys Res A Accelerators Spectrometers Detectors Assoc Equip. 2017;874:43–9.
Sandler N, Määttänen A, Ihalainen P, Kronberg L, Meierjohann A, Viitala T, et al. Inkjet printing of drug substances and use of porous substrates-towards individualized dosing. J Pharm Sci. 2011;100:3386–95.
Ahn S-H, And S, Wright P, Montero M, Odell D, Roundy S. Anisotropic material properties of fused deposition modeling ABS. Rapid Prototyp J. 2002;8:248–57.
Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of five-in-one dose combination polypill with defined immediate and sustained release profiles. J Control Release. 2015;217:308–14.
Cerda JR, Arifi T, Ayyoubi S, Knief P, Ballesteros MP, Keeble W, et al. Personalised 3D Printed Medicines: Optimising Material Properties for Successful Passive Diffusion Loading of Filaments for Fused Deposition Modelling of Solid Dosage Forms. Pharmaceutics. 2020;12:345.
Goyanes A, Allahham N, Trenfield SJ, Stoyanov E, Gaisford S, Basit AW. Direct powder extrusion 3D printing: fabrication of drug products using a novel single-step process. Int J Pharm. 2019;567:118471.
El Aita I, Breitkreutz J, Quodbach J. On-demand manufacturing of immediate release levetiracetam tablets using pressure-assisted microsyringe printing. Eur J Pharm Biopharm. 2019;134:29–36.
Gooleand J, Amighi K. 3D printing in pharmaceutics: a new tool for designing customized drug delivery systems. Int J Pharm. 2016;499:376–94.
Carveand M, Wlodkowic D. 3D-Printed Chips: Compatibility of Additive Manufacturing Photopolymeric Substrata with Biological Applications. Micromachines. 2018;9:91.
Toxicological evaluation of benzophenone. Scientific Opinion of the Panel on food contact materials, enzymes, flavourings and processing aids (CEF). Retrieved the 10 Mya 2020 from: (2009) https://doi.org/10.2903/j.efsa.2009.1104.
Clear Photoreactive Resin for Formlabs 3D Printers. Retrieved the 10 May 2020 from: https://formlabs.com/media/upload/Clear__Resin_SDS_EU.pdf.
Khot S. A review on powder bed fusion technology of metal additive manufacturing. (2017).
Kruth JP, Mercelis P, Vaerenbergh J, Froyen L, Rombouts M. Binding mechanisms in selective laser sintering and selective laser melting. Rapid Prototyp J. 2005;11:26–36.
Fina F, Goyanes A, Gaisford S, Basit AW. Selective laser sintering (SLS) 3D printing of medicines. Int J Pharm. 2017;529:285–93.
Candurin® Gold Sheen Safety Technical Data Sheet. Retrieved the 11 May 2020 form: file:///C:/Users/serra/Downloads/120608_SDS_GB_EN%20(1).PDF.
Khaled SA, Burley JC, Alexander MR, Yang J, Roberts CJ. 3D printing of tablets containing multiple drugs with defined release profiles. Int J Pharm. 2015;494:643–50.
Fakhari A, Corcoran M, Schwarz A. Thermogelling properties of purified poloxamer 407. Heliyon. 2017;3:26.
Haring AP, Tong Y, Halper J, Johnson BN. Programming of multicomponent temporal release profiles in 3D printed Polypills via Core-Shell, multilayer, and gradient concentration profiles. Adv Healthc Mater. 2018;7:1–10.
Pereira BC, Isreb A, Forbes RT, Dores F, Habashy R, Petit JB, et al. 'Temporary Plasticiser': a novel solution to fabricate 3D printed patient-centred cardiovascular 'Polypill' architectures. Eur J Pharm Biopharm. 2019;135:94–103.
Genina N, Boetker JP, Colombo S, Harmankaya N, Rantanen J, Bohr A. Anti-tuberculosis drug combination for controlled oral delivery using 3D printed compartmental dosage forms: from drug product design to in vivo testing. J Control Release. 2017;268:40–8.
Gioumouxouzis CI, Baklavaridis A, Katsamenis OL, Markopoulou CK, Bouropoulos N, Tzetzis D, et al. A 3D printed bilayer oral solid dosage form combining metformin for prolonged and glimepiride for immediate drug delivery. Eur J Pharm Sci. 2018;120:40–52.
Goyanes A, Wang J, Buanz A, Martínez-Pacheco R, Telford R, Gaisford S, et al. 3D printing of medicines: engineering novel Oral devices with unique design and drug release characteristics. Mol Pharm. 2015;12:4077–84.
Rycerz K, Stepien KA, Czapiewska M, Arafat BT, Habashy R, Isreb A, et al. Embedded 3D printing of novel bespoke soft dosage form concept for pediatrics. Pharmaceutics. 2019;11:630.
Trenfield SJ, Tan HX, Goyanes A, Wilsdon D, Rowland M, Gaisford S, et al. Non-destructive dose verification of two drugs within 3D printed polyprintlets. Int J Pharm. 2020;577:119066.
Awad A, Fina F, Trenfield SJ, Patel P, Goyanes A, Gaisford S, et al. 3D printed pellets (Miniprintlets): a novel, multi-drug, Controlled Release Platform Technology. Pharmaceutics. 2019;11:148.
Robles-Martinez P, Xu X, Trenfield SJ, Awad A, Goyanes A, Telford R, et al. 3D Printing of a Multi-Layered Polypill Containing Six Drugs Using a Novel Stereolithographic Method. Pharmaceutics. 2019;11:274.
Xu X, Robles-Martinez P, Madla CM, Joubert F, Goyanes A, Basit AW, et al. Stereolithography (SLA) 3D printing of an antihypertensive polyprintlet: case study of an unexpected photopolymer-drug reaction. Addit Manuf. 2020;33:101071.
Park BJ, Choi HJ, Moon SJ, Kim SJ, Bajracharya R, Min JY, et al. Pharmaceutical applications of 3D printing technology: current understanding and future perspectives. J Pharma Investig. 2019;49:575–85.
AEMPS. CIMA - Centro de información online de medicamentos de la AEMPS, Vol. 2019, AEMPS, AEMPS, 2017.
Park K. 3D printing of 5-drug polypill. J Control Release. 2015;217:352.
Padhee KK, Patel NS, Koduri SC, Mukharya A, Modi IA, Modi RI, and Khamar BM. Stable pharmaceutical composition for atherosclerosis. In C.P. Ltd (ed.), Vol. EP395838B12010.
Pereira B, Isreb A, Forbes R, Dores F, Habashy R, Petit J-B, et al. ‘Temporary Plasticiser’: A Novel Solution to Fabricate 3D Printed Patient-Centred Cardiovascular ‘Polypill’ Architectures. Eur J Pharm Biopharm. 2018;135:94–103.
Haring AP, Tong Y, Halper J, Johnson BN. Programming of multicomponent temporal release profiles in 3D printed Polypills via Core–Shell, multilayer, and gradient concentration profiles. Adv Healthc Mater. 2018;7:1800213.
Konta AA, García-Piña M, Serrano DR. Personalised 3D printed medicines: which techniques and polymers are more successful? Bioengineering (Basel). 2017;4(79).
Lu Y, Mantha SN, Crowder DC, Chinchilla S, Shah KN, Yun YH, et al. Microstereolithography and characterization of poly(propylene fumarate)-based drug-loaded microneedle arrays. Biofabrication. 2015;7:045001.
Branciforti DS, Lazzaroni S, Milanese C, Castiglioni M, Auricchio F, Pasini D, et al. Visible light 3D printing with epoxidized vegetable oils. Addit Manuf. 2019;25:317–24.
Acknowledgments and Disclosures
This project was funded by a UCM-Santander University project (PR26/16-20355) to F. Bolás-Fernández. Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fernández-García, R., Prada, M., Bolás-Fernández, F. et al. Oral Fixed-Dose Combination Pharmaceutical Products: Industrial Manufacturing Versus Personalized 3D Printing. Pharm Res 37, 132 (2020). https://doi.org/10.1007/s11095-020-02847-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02847-3