Abstract
Purpose
Therapeutic antibodies have heterogeneities in their structures, although its structural alteration in the body is unclear. Here, we analyzed the change of amino acid modifications and carbohydrate chains of rituximab after administration to patients.
Methods
Twenty B cell non-Hodgkin’s lymphoma patients who were treated with rituximab for the first time or after more than one year’s abstinence were recruited. Structural analysis of rituximab was carried out at 1 h after administration and at the trough by using liquid chromatography/time-of-flight-mass spectrometry. Plasma rituximab concentration and pharmacodynamic markers were also determined.
Results
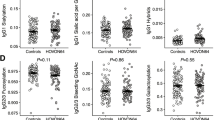
Of recruited twenty, 3 patients exhibited rapid rituximab clearance. Nine types of carbohydrate chains were detected in rituximab isolated from the blood. The composition ratios in some glycoforms were significantly different between at 1 h after administration and at the trough, although consisted amino acids remained unchanged. The patients with high clearance showed extensive alterations of glycoform composition ratios. However, pharmacodynamics makers were not different.
Conclusion
Inter-individual variations in plasma concentrations of rituximab were found in some B-NHL patients. We could analyze a change in glycoforms of rituximab in the patients, and this finding may affect the pharmacokinetics of rituximab.






Similar content being viewed by others
Abbreviations
- ADCC:
-
Antibody-dependent cellular cytotoxicity
- B-NHL:
-
B cell non-Hodgkin’s lymphoma
- CHOP:
-
Cyclophosphamide, doxorubicin, vincristine and prednisone
- DLBCL:
-
Diffuse large B cell lymphoma
- ELISA:
-
Enzyme-linked immunosorbent assay
- Fab:
-
Fragment antigen-binding
- Fc:
-
Fragment crystallizable
- FL:
-
Follicular lymphoma
- LC/TOF-MS:
-
Liquid chromatograph time-of-flight mass spectrometer
- mAb:
-
Monoclonal antibody
- NK cell:
-
Natural killer cell
- nSMOL:
-
Nano-Surface and Molecular-Orientation Limited
- PBMC:
-
Peripheral blood mononuclear cell
- R-CHOP:
-
CHOP chemotherapy in combination with rituximab
- R-CVP:
-
Cyclophosphamide, vincristine and prednisone chemotherapy in combination with rituximab
- sIL-2R:
-
Soluble Interleukin-2 receptor.
References
Chan AC, Carter PJ. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10:301–16.
Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341:1192–8.
Newsome BW, Ernstoff MS. The clinical pharmacology of therapeutic monoclonal antibodies in the treatment of malignancy; have the magic bullets arrived? Br J Clin Pharmacol. 2008;66:6–19.
Yamane-Ohnuki N, Satoh M. Production of therapeutic antibodies with controlled fucosylation. MAbs. 2009;1:230–6.
Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, et al. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–73.
Chung S, Quarmby V, Gao X, Ying Y, Lin L, Reed C, et al. Quantitative evaluation of fucose reducing effects in a humanized antibody on Fcγ receptor binding and antibody-dependent cell-mediated cytotoxicity activities. MAbs. 2012;4:326–40.
Herter S, Herting F, Mundigl O, Waldhauer I, Weinzierl T, Fauti T, et al. Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther. 2013;12:2031–42.
Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370:1101–10.
Hodoniczky J, Zheng YZ, James DC. Control of recombinant monoclonal antibody effector functions by fc N-glycan remodeling in vitro. Biotechnol Prog. 2005;21:1644–52.
Liu YD, Flynn GC. Effect of high mannose glycan pairing on IgG antibody clearance. Biologicals. 2016;44:163–9.
Alessandri L, Ouellette D, Acquah A, Rieser M, Leblond D, Saltarelli M, et al. Increased serum clearance of oligomannose species present on a human IgG1 molecule. MAbs. 2012;4:509–20.
Otani Y, Yonezawa A, Tsuda M, Imai S, Ikemi Y, Nakagawa S, et al. Time-dependent structural alteration of rituximab analyzed by LC/TOF-MS after a systemic administration to rats. PLoS One. 2017;12:e0169588.
Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–42.
Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116:2040–5.
Pfreundschuh M, Schubert J, Ziepert M, Schmits R, Mohren M, Lengfelder E, Reiser M, Nickenig C, Clemens M, Peter N, Bokemeyer C, Eimermacher H, Ho A, Hoffmann M, Mertelsmann R, Trümper L, Balleisen L, Liersch R, Metzner B, Hartmann F, Glass B, Poeschel V, Schmitz N, Ruebe C, Feller AC, Loeffler M, German High-Grade Non-Hodgkin Lymphoma Study Group (DSHNHL). Six versus eight cycles of bi-weekly CHOP-14 with or without rituximab in elderly patients with aggressive CD20+ B-cell lymphomas: a randomised controlled trial (RICOVER-60). Lancet Oncol 2008; 9:105–116.
Pfreundschuh M, Trumper L, Osterborg A, Pettengell R, Trneny M, Imrie K, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera international trial (MInT) group. Lancet Oncol. 2006;7:379–91.
Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–7.
Horn J, Kleber M, Hieke S, Schmitt-Graff A, Wasch R, Engelhardt M. Treatment option of bendamustine in combination with rituximab in elderly and frail patients with aggressive B-non-Hodgkin lymphoma: rational, efficacy, and tolerance. Ann Hematol. 2012;91:1579–86.
Henry C, Deschamps M, Rohrlich PS, Pallandre JR, Remy-Martin JP, Callanan M, et al. Identification of an alternative CD20 transcript variant in B-cell malignancies coding for a novel protein associated to rituximab resistance. Blood. 2010;115:2420–9.
Iwamoto N, Shimada T, Umino Y, Aoki C, Aoki Y, Sato TA, et al. Selective detection of complementarity-determining regions of monoclonal antibody by limiting protease access to the substrate: nano-surface and molecular-orientation limited proteolysis. Analyst. 2014;139:576–80.
Iwamoto N, Takanashi M, Hamada A, Shimada T. Validated LC/MS bioanalysis of rituximab CDR peptides using Nano-surface and Molecular-orientation limited (nSMOL) proteolysis. Biol Pharm Bull. 2016;39:1187–94.
Dua P, Hawkins E, van der Graaf PH. A tutorial on target-mediated drug disposition (TMDD) models. CPT Pharmacometrics Syst Pharmacol. 2015;4:324–37.
Deaglio S, Zubiaur M, Gregorini A, Bottarel F, Ausiello CM, Dianzani U, et al. Human CD38 and CD16 are functionally dependent and physically associated in natural killer cells. Blood. 2002;99:2490–8.
Hampson G, Ward TH, Cummings J, Bayne M, Tutt AL, Cragg MS, et al. Validation of an ELISA for the determination of rituximab pharmacokinetics in clinical trials subjects. J Immunol Methods. 2010;360:30–8.
Cragg MS, Bayne MB, Tutt AL, French RR, Beers S, Glennie MJ, et al. A new anti-idiotype antibody capable of binding rituximab on the surface of lymphoma cells. Blood. 2004;104:2540–2.
Dostalek M, Gardner I, Gurbaxani BM, Rose RH, Chetty M. Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies. Clin Pharmacokinet. 2013;52:83–124.
Garg A, Balthasar JP. Physiologically-based pharmacokinetic (PBPK) model to predict IgG tissue kinetics in wild-type and FcRn-knockout mice. J Pharmacokinet Pharmacodyn. 2007;34:687–709.
Schiestl M, Stangler T, Torella C, Cepeljnik T, Toll H, Grau R. Acceptable changes in quality attributes of glycosylated biopharmaceuticals. Nat Biotechnol. 2011;29:310–2.
Counsilman CE, Jol-van der Zijde CM, Stevens J, Cransberg K, Bredius RG, Sukhai RN. Pharmacokinetics of rituximab in a pediatric patient with therapy-resistant nephrotic syndrome. Pediatr Nephrol. 2015;30:1367–70.
Stroh M, Winter H, Marchand M, Claret L, Eppler S, Ruppel J, et al. Clinical pharmacokinetics and pharmacodynamics of Atezolizumab in metastatic urothelial carcinoma. Clin Pharmacol Ther. 2017;102:305–12.
Lioger B, Edupuganti SR, Mulleman D, Passot C, Desvignes C, Bejan-Angoulvant T, et al. Antigenic burden and serum IgG concentrations influence rituximab pharmacokinetics in rheumatoid arthritis patients. Br J Clin Pharmacol. 2017;83:1773–81.
Eser A, Primas C, Reinisch W. Drug monitoring of biologics in inflammatory bowel disease. Curr Opin Gastroenterol. 2013;29:391–6.
Montoyo HP, Vaccaro C, Hafner M, Ober RJ, Mueller W, Ward ES. Conditional deletion of the MHC class I-related receptor FcRn reveals the sites of IgG homeostasis in mice. Proc Natl Acad Sci U S A. 2009;106:2788–93.
Arnold JN, Wormald MR, Sim RB, Rudd PM, Dwek RA. The impact of glycosylation on the biological function and structure of human immunoglobulins. Annu Rev Immunol. 2007;25:21–50.
van Bilsen K, van Hagen PM, Bastiaans J, van Meurs JC, Missotten T, Kuijpers RW, et al. The neonatal fc receptor is expressed by human retinal pigment epithelial cells and is downregulated by tumour necrosis factor-alpha. Br J Ophthalmol. 2011;95:864–8.
Cervenak J, Doleschall M, Bender B, Mayer B, Schneider Z, Doleschall Z, et al. NFkappaB induces overexpression of bovine FcRn: a novel mechanism that further contributes to the enhanced immune response in genetically modified animals carrying extra copies of FcRn. MAbs. 2013;5:860–71.
Maloney DG, Liles TM, Czerwinski DK, Waldichuk C, Rosenberg J, Grillo-Lopez A, et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood. 1994;84:2457–66.
Maloney DG, Grillo-Lopez AJ, Bodkin DJ, White CA, Liles TM, Royston I, et al. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma. J Clin Oncol. 1997;15:3266–74.
Tout M, Casasnovas O, Meignan M, Lamy T, Morschhauser F, Salles G, et al. Rituximab exposure is influenced by baseline metabolic tumor volume and predicts outcome of DLBCL patients: a lymphoma study association report. Blood. 2017;129:2616–23.
Carella AM, de Souza CA, Luminari S, Marcheselli L, Chiappella A, di Rocco A, et al. Prognostic role of gender in diffuse large B-cell lymphoma treated with rituximab containing regimens: a Fondazione Italiana Linfomi/Grupo de Estudos em Molestias Onco-Hematologicas retrospective study. Leuk Lymphoma. 2013;54:53–7.
Rozman S, Grabnar I, Novakovic S, Mrhar A, Jezersek Novakovic B. Population pharmacokinetics of rituximab in patients with diffuse large B-cell lymphoma and association with clinical outcome. Br J Clin Pharmacol. 2017;83:1782–90.
Acknowledgments and Disclosures
This work was supported by the Research on Regulatory Harmonization and Evaluation of Pharmaceuticals, Medical Devices, Regenerative and Cellular Therapy Products, Gene Therapy Products, and Cosmetics from the Japan Agency for Medical Research and development, AMED, the Japan Research Foundation for Clinical Pharmacology, and the Mochida Memorial Foundation for Medical and Pharmaceutical Research to A.Y. The authors are grateful to all the medical staff of Department of Hematology, Kyoto University Hospital. All authors have no conflicts of interest to declare. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study protocol was approved by the Ethics Committee of Kyoto University Graduate School and Faculty of Medicine and Kyoto University Hospital (E2472), and was registered at the University Hospital Medical Information Network-Clinical Trial Registry System (UMIN000016713). Written informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yonezawa, A., Otani, Y., Kitano, T. et al. Concentration and Glycoform of Rituximab in Plasma of Patients with B Cell Non-Hodgkin’s Lymphoma. Pharm Res 36, 82 (2019). https://doi.org/10.1007/s11095-019-2624-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2624-5