Abstract
Purpose
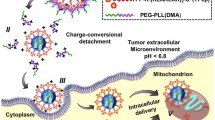
The present study is aimed at designing an appropriate co-delivery system for chemotherapeutic drugs and gene drugs with high loading capacity, on-demand release behaviors, efficient endosomal escape, and enhanced nucleic localization, thereby providing efficacious antitumor activity.
Methods
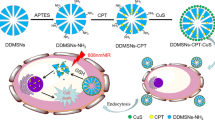
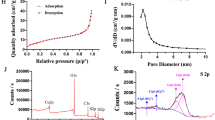
Schiff-base linked imidazole dendritic mesoporous silica nanoparticles (SL-IDMSN) were developed and employed to load doxorubicin (DOX) and survivin shRNA-expressing plasmid (iSur-pDNA) to form nanocomplexes. The nanoparticles were assessed by structural characterization, drug loading and release, cellular uptake, intracellular distribution, gene transfection, in vitro anti-proliferation of hepatoma cells, and in vivo tumor growth inhibition in H-22 tumor bearing mice.
Results
SL-IDMSN showed high loading capacity for both DOX and iSur-pDNA due to their hierarchical mesostructures. The cleavage of Schiff-base linkage on SL-IDMSN in the weakly acidic endosomes/lysosomes led to microenvironment-specific release of both DOX and iSur-pDNA. Meanwhile, the imidazole modification could trigger the efficient endosomal escape via proton sponge effect, thereby enhancing nuclear accumulation of iSur-pDNA and gene silencing efficiency. More importantly, these superior performances of SL-IDMSN resulted in their improved inhibitory effects on in vitro cancer cell proliferation and in vivo tumor growth.
Conclusions
SL-IDMSN is a microenvironment-sensitive and biocompatible nanocarrier for the co-delivery of DOX and iSur-pDNA, which might be a promising carrier for co-delivery of chemotherapeutic drugs and gene drugs for synergistic cancer therapy.








Similar content being viewed by others
Abbreviations
- APTES:
-
(3-aminopropy) triethoxysilane
- BET:
-
Brunauer-Emmett-Teller
- CLSM:
-
Confocal laser scanning microscopy
- CTAB:
-
Cetyltrimethylammonium bromide
- DL:
-
Drug loading
- DMEM:
-
Dulbecco’s modified eagle medium
- DMSN:
-
Dendrimer mesoporous silica nanoparticles
- DMSN@DOX:
-
DOX loaded DMSN nanoparticles
- DMSN@DOX/pDNA:
-
iSur-pDNA loaded DMSN@DOX nanocomplexes
- DOX:
-
Doxorubicin
- FT-IR:
-
Fourier transform-infrared spectroscopy
- FITC-pDNA:
-
FITC-labeled pDNA
- IC50 :
-
Half maximal inhibitory concentration
- iSur-pDNA:
-
Survivin shRNA-expressing plasmid
- MTT:
-
Methyl thiazolyl tetrazolium
- PBS:
-
Phosphate buffer solution
- SAXS:
-
Small-angle X-ray scattering
- SDS:
-
Sodium dodecyl sulfate
- SL-IDMSN:
-
Schiff-base linked imidazole dendritic mesoporous silica nanoparticles
- SL-IDMSN@DOX:
-
DOX loaded SL-IDMSN nanoparticles
- SL-IDMSN@DOX/pDNA:
-
iSur-pDNA loaded SL-IDMSN@DOX nanocomplexes
- TEA:
-
Triethanolamine
- TEM:
-
Transmission electron microscopy
- TEOS:
-
Tetraethyl orthosilicate
- TIR:
-
Tumor inhibition ratio
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):359–86.
He Q, Gao Y, Zhang L, Zhang Z, Gao F, Ji X, et al. A pH-responsive mesoporous silica nanoparticles-based multi-drug delivery system for overcoming multi-drug resistance. Biomaterials. 2011;32(30):7711–20.
Blum AP, Kammeyer JK, Rush AM, Callmann CE, Hahn ME, Gianneschi NC. Stimuli-responsive nanomaterials for biomedical applications. J Am Chem Soc. 2015;137(6):2140–54.
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings1. Adv Drug Deliv Rev. 2001;46(1–3):3–26.
Jhaveri A, Deshpande P, Torchilin V. Stimuli-sensitive nanopreparations for combination cancer therapy. J Control Release. 2014;190:352–70.
Wang Y, Gao S, Ye W-H, Yoon HS, Yang Y-Y. Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nat Mater. 2006;5(10):791–6.
Greco F, Vicent MJ. Combination therapy: opportunities and challenges for polymer-drug conjugates as anticancer nanomedicines. Adv Drug Deliv Rev. 2009;61(13):1203–13.
Li C, Yang D. Ma pa, Chen Y, Wu Y, Hou Z, et al. multifunctional Upconversion mesoporous silica nanostructures for dual modal imaging and in vivo drug delivery. Small. 2013;9(24):4150–9.
Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–82.
Du X, Shi B, Liang J, Bi J, Dai S, Qiao SZ. Developing functionalized dendrimer-like silica nanoparticles with hierarchical pores as advanced delivery nanocarriers. Adv Mater. 2013;25(41):5981–5.
Slowing II, Vivero-Escoto JL, Wu CW, Lin VSY. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev. 2008;60(11):1278–88.
Yang P, Gai S, Lin J. Functionalized mesoporous silica materials for controlled drug delivery. Chem Soc Rev. 2012;41(9):3679–98.
Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24(12):1504–34.
Tian L, Bae YH. Cancer nanomedicines targeting tumor extracellular pH. Colloid Surface B. 2012;99:116–26.
Yan L, Zhang J, Lee C-S, Chen X. Micro- and nanotechnologies for intracellular delivery. Small. 2014;10(22):4487–504.
Tian HY, Chen J, Chen XS. Nanoparticles for gene delivery. Small. 2013;9(12):2034–44.
Xu S, Olenyuk BZ, Okamoto CT, Hamm-Alvarez SF. Targeting receptor-mediated endocytotic pathways with nanoparticles: rationale and advances. Adv Drug Deliv Rev. 2013;65(1):121–38.
Xin Y, Yuan J. Schiff's base as a stimuli-responsive linker in polymer chemistry. Polym Chem. 2012;3(11):3045–55.
Bae Y, Fukushima S, Harada A, Kataoka K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew Chem Int Ed. 2003;42(38):4640–3.
Shen D, Yang J, Li X, Zhou L, Zhang R, Li W, et al. Biphase stratification approach to three-dimensional dendritic biodegradable mesoporous silica nanospheres. Nano Lett. 2014;14(2):923–32.
Slowing I, Trewyn BG, Lin VSY. Effect of surface functionalization of MCM-41-type mesoporous silica nanoparticleson the endocytosis by human cancer cells. J Am Chem Soc. 2006;128(46):14792–3.
Legras A, Kondor A, Heitzmann MT, Truss RW. Inverse gas chromatography for natural fibre characterisation: identification of the critical parameters to determine the Brunauer-Emmett-teller specific surface area. J Chromatogr A. 2015;1425:273–9.
Villarroel-Rocha J, Barrera D, Sapag K. Introducing a self-consistent test and the corresponding modification in the Barrett, Joyner and Halenda method for pore-size determination. Microporous Mesoporous Mat. 2014;200:68–78.
Sun L, Deng XH, Yang X, Li ZJ, Wang ZH, Li L, et al. Co-delivery of doxorubicin and curcumin by polymeric micelles for improving antitumor efficacy on breast carcinoma. RSC Adv. 2014;4(87):46737–50.
Shi B, Zhang H, Dai S, Du X, Bi J, Qiao SZ. Intracellular microenvironment responsive polymers: a multiple-stage transport platform for high-performance gene delivery. Small. 2014;10(5):871–7.
Yu B, Tang C, Yin C. Enhanced antitumor efficacy of folate modified amphiphilic nanoparticles through co-delivery of chemotherapeutic drugs and genes. Biomaterials. 2014;35(24):6369–78.
He C, Hu Y, Yin L, Tang C, Yin C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials. 2010;31(13):3657–66.
Mesa MM, Macias M, Cantero D. A simplification of the protein assay method of Ramsay et al. for the quantification of Thiobacillus ferrooxidans in the presence of ferric precipitates. Appl Microbiol Biot. 2000;53(6):722–5.
Zheng H, Tang C, Yin CH. The effect of crosslinking agents on the transfection efficiency, cellular and intracellular processing of DNA/polymer nanocomplexes. Biomaterials. 2013;34(13):3479–88.
Douglas KL, Piccirillo CA, Tabrizian M. Cell line-dependent internalization pathways and intracellular trafficking determine transfection efficiency of nanoparticle vectors. Eur J Pharm Biopharm. 2008;68(3):676–87.
Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58(1):32–45.
Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem J. 2004;377(1):159–69.
Shi J, Guobao W, Chen H, Zhong W, Qiu X, Xing MMQ. Schiff based injectable hydrogel for in situ pH-triggered delivery of doxorubicin for breast tumor treatment. Polym Chem. 2014;5(21):6180–9.
Gao F, Botella P, Corma A, Blesa J, Dong L. Monodispersed mesoporous silica nanoparticles with very large pores for enhanced adsorption and release of DNA. J Phys Chem B. 2009;113(6):1796–804.
Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007;59(8):748–58.
Mrkvan T, Sirova M, Etrych T, Chytil P, Strohalm J, Plocova D, et al. Chemotherapy based on HPMA copolymer conjugates with pH-controlled release of doxorubicin triggers anti-tumor immunity. J Control Release. 2005;110(1):119–29.
Pearce TR, Shroff K, Kokkoli E. Peptide targeted lipid nanoparticles for anticancer drug delivery. Adv Mater. 2012;24(28):3803–22.
Mishra S, Heidel JD, Webster P, Davis ME. Imidazole groups on a linear, cyclodextrin-containing polycation produce enhanced gene delivery via multiple processes. J Control Release. 2006;116(2):179–91.
Altieri DC. Targeting survivin in cancer. Cancer Lett. 2013;332(2):225–8.
Habib R, Akhtar J, Taqi M, Yu C, Zhang C. Lentiviral vector-mediated survivin shRNA delivery in gastric cancer cell lines significantly inhibits cell proliferation and tumor growth. Oncol Rep. 2015;34(2):859–67.
Shoeneman JK, Ehrhart EJ, Eickhoff JC, Charles JB, Powers BE, Thamm DH. Expression and function of survivin in canine osteosarcoma. Cancer Res. 2012;72(1):249–59.
Sarela AI, Verbeke CS, Ramsdale J, Davies CL, Markham AF, Guillou PJ. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Brit J Cancer. 2002;86(6):886–92.
Acknowledgments and Disclosures
The authors are thankful for the financial support from the National Natural Science Foundation of China (No. 81273460).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 900 kb)
Rights and permissions
About this article
Cite this article
Li, Z., Zhang, L., Tang, C. et al. Co-Delivery of Doxorubicin and Survivin shRNA-Expressing Plasmid Via Microenvironment-Responsive Dendritic Mesoporous Silica Nanoparticles for Synergistic Cancer Therapy. Pharm Res 34, 2829–2841 (2017). https://doi.org/10.1007/s11095-017-2264-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2264-6