Abstract
Purpose
Paclitaxel (PTX) is currently used in combination with cisplatin for Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for the treatment of peritoneal carcinomatosis. Albumin-bound PTX is a promising new drug for HIPEC because of its easy solubility in aqueous perfusion medium and possibly because of the tendency of albumin to cross physiological barriers and accumulate in tumor tissue.
Methods
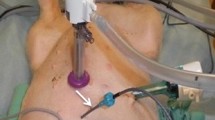
We tested the feasibility of using nab-paclitaxel in rabbits treated by HIPEC for 60 min compared with the classical formulation at an equivalent PTX dose. Samples of perfusate and blood were collected at different time points and peritoneal tissues were collected at the end of perfusion. PTX concentrations were determined by HPLC. The depth of paclitaxel penetration through the peritoneal barrier was assessed by mass spectrometry imaging.
Results
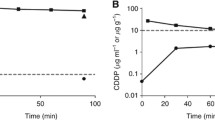
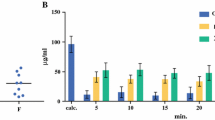
PTX after nab-paclitaxel treatment penetrated up to 0.63 mm in the peritoneal wall, but after CRE-paclitaxel, it was not detectable in the peritoneum. Moreover, the peritoneal concentration after nab-paclitaxel was five times that after paclitaxel classical formulation. Despite the high levels reached in the peritoneum, systemic exposure of PTX was low.
Conclusions
Our results show that nab-paclitaxel penetrates into the abdominal wall better than CRE-paclitaxel, in terms of effective penetration and peritoneal tissue concentration.




Similar content being viewed by others
Abbreviations
- CRE:
-
CremophorEL
- EPR:
-
Enhanced permeation and retention
- HIPEC:
-
Hyperthermic intraperitoneal chemotherapy
- IS:
-
Internal standard
- LOQ:
-
Limit of quantitation
- MALDI:
-
Matrix assisted laser desorption ionization
- MP:
-
Mobile phase
- MSI:
-
Mass spectrometry imaging
- Nab :
-
Nanoparticles-albumin-bound
- PTX:
-
Paclitaxel
- ROI:
-
Region of interest
References
Coccolini F, Cotte E, Glehen O, Lotti M, Poiasina E, Catena F, et al. Intraperitoneal chemotherapy in advanced gastric cancer. Meta-analysis of randomized trials. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2014;40(1):12–26.
Coccolini F, Gheza F, Lotti M, Virzì S, Iusco D, Ghermandi C, et al. Peritoneal carcinomatosis. World J Gastroenterol. 2013;19(41):6979–94.
Sugarbaker PH, Van der Speeten K. Surgical technology and pharmacology of hyperthermic perioperative chemotherapy. J Gastrointest Oncol. 2016;7(1):29–44.
Ansaloni L, Coccolini F, Morosi L, Ballerini A, Ceresoli M, Grosso G, et al. Pharmacokinetics of concomitant cisplatin and paclitaxel administered by hyperthermic intraperitoneal chemotherapy to patients with peritoneal carcinomatosis from epithelial ovarian cancer. Br J Cancer. 2015;112(2):306–12.
de Bree E, Rosing H, Filis D, Romanos J, Melisssourgaki M, Daskalakis M, et al. Cytoreductive surgery and intraoperative hyperthermic intraperitoneal chemotherapy with paclitaxel: a clinical and pharmacokinetic study. Ann Surg Oncol. 2008;15(4):1183–92.
Emoto S, Sunami E, Yamaguchi H, Ishihara S, Kitayama J, Watanabe T. Drug development for intraperitoneal chemotherapy against peritoneal carcinomatosis from gastrointestinal cancer. Surg Today. 2014;44(12):2209–20.
Soma D, Kitayama J, Ishigami H, Kaisaki S, Nagawa H. Different tissue distribution of paclitaxel with intravenous and intraperitoneal administration. J Surg Res. 2009;155(1):142–6.
Markman M. Intraperitoneal antineoplastic drug delivery: rationale and results. Lancet Oncol. 2003;4(5):277–83.
Simón-Gracia L, Hunt H, Scodeller PD, Gaitzsch J, Braun GB, Willmore A-MA, et al. Paclitaxel-loaded Polymersomes for enhanced intraperitoneal chemotherapy. Mol Cancer Ther. 2016;15(4):670–9.
Coccolini F, Campanati L, Catena F, Ceni V, Ceresoli M, Jimenez Cruz J, et al. Hyperthermic intraperitoneal chemotherapy with cisplatin and paclitaxel in advanced ovarian cancer: a multicenter prospective observational study. J Gynecol Oncol. 2015;26(1):54–61.
de Bree E. Optimal drugs for HIPEC in different tumors. J BUON Off J Balk Union Oncol. 2015;20(Suppl 1):S40–6.
Braam HJ, Schellens JH, Boot H, van Sandick JW, Knibbe CA, Boerma D, et al. Selection of chemotherapy for hyperthermic intraperitoneal use in gastric cancer. Crit Rev Oncol Hematol. 2015;95(3):282–96.
Liu L-L, Yi T, Zhao X. Antitumor effect of D-erythrose in an abdominal metastatic model of colon carcinoma. Oncol Lett. 2015;9(2):769–73.
Mehta AM, Van den Hoven JM, Rosing H, Hillebrand MJX, Nuijen B, Huitema ADR, et al. Stability of oxaliplatin in chloride-containing carrier solutions used in hyperthermic intraperitoneal chemotherapy. Int J Pharm. 2015;479(1):23–7.
Kundranda MN, Niu J. Albumin-bound paclitaxel in solid tumors: clinical development and future directions. Drug Design, Development and Therapy. 2015;9:3767–77.
Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Control Release Off J Control Release Soc. 2013;170(3):365–72.
Yu X, Jin C. Application of albumin-based nanoparticles in the management of cancer. J Mater Sci Mater Med. 2016;27(1):4.
Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010;102(11):1555–77.
Morosi L, Zucchetti M, D’Incalci M, Davoli E. Imaging mass spectrometry: challenges in visualization of drug distribution in solid tumors. Curr Opin Pharmacol. 2013;13(5):807–12.
Morosi L, Spinelli P, Zucchetti M, Pretto F, Carrà A, D’Incalci M, et al. Determination of paclitaxel distribution in solid tumors by nano-particle assisted laser desorption ionization mass spectrometry imaging. PloS One. 2013;8(8):e72532.
Fruscio R, Lissoni AA, Frapolli R, Corso S, Mangioni C, D’Incalci M, et al. Clindamycin-paclitaxel pharmacokinetic interaction in ovarian cancer patients. Cancer Chemother Pharmacol. 2006;58(3):319–25.
Cesca M, Frapolli R, Berndt A, Scarlato V, Richter P, Kosmehl H, et al. The effects of vandetanib on paclitaxel tumor distribution and antitumor activity in a xenograft model of human ovarian carcinoma. Neoplasia N Y N. 2009;11(11):1155–64.
Bouquet W, Deleye S, Staelens S, De Smet L, Van Damme N, Debergh I, et al. Antitumour efficacy of two paclitaxel formulations for hyperthermic intraperitoneal chemotherapy (HIPEC) in an in vivo rat model. Pharm Res. 2011;28(7):1653–60.
Eiseman JL, Eddington ND, Leslie J, MacAuley C, Sentz DL, Zuhowski M, et al. Plasma pharmacokinetics and tissue distribution of paclitaxel in CD2F1 mice. Cancer Chemotherapy and Pharmacology. 1994;34(6):465–71.
Colombo C, Morosi L, Bello E, Ferrari R, Licandro SA, Lupi M, et al. PEGylated Nanoparticles Obtained through Emulsion Polymerization as Paclitaxel Carriers. Mol Pharm. 2016;13(1):40–6.
Wei Y, Xue Z, Ye Y, Wang P, Huang Y, Zhao L. Pharmacokinetic and tissue distribution of paclitaxel in rabbits assayed by LC-UV after intravenous administration of its novel liposomal formulation. Biomed Chromatogr BMC. 2014;28(2):204–12.
Ansaloni L, Agnoletti V, Amadori A, Catena F, Cavaliere D, Coccolini F, et al. Evaluation of extensive cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer Off J Int Gynecol Cancer Soc. 2012;22(5):778–85.
Muñoz-Casares FC, Rufián S, Arjona-Sánchez Á, Rubio MJ, Díaz R, Casado Á, et al. Neoadjuvant intraperitoneal chemotherapy with paclitaxel for the radical surgical treatment of peritoneal carcinomatosis in ovarian cancer: a prospective pilot study. Cancer Chemother Pharmacol. 2011;68(1):267–74.
Muñoz-Casares FC, Rufián S, Rubio MJ, Díaz CJ, Díaz R, Casado A, et al. The role of hyperthermic intraoperative intraperitoneal chemotherapy (HIPEC) in the treatment of peritoneal carcinomatosis in recurrent ovarian cancer. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex. 2009;11(11):753–9.
Kim JH, Lee JM, Ryu KS, Lee YS, Park YG, Hur SY, et al. Consolidation hyperthermic intraperitoneal chemotherapy using paclitaxel in patients with epithelial ovarian cancer. J Surg Oncol. 2010;101(2):149–55.
Bae JH, Lee JM, Ryu KS, Lee YS, Park YG, Hur SY, et al. Treatment of ovarian cancer with paclitaxel- or carboplatin-based intraperitoneal hyperthermic chemotherapy during secondary surgery. Gynecol Oncol. 2007;106(1):193–200.
Rufián S, Muñoz-Casares FC, Briceño J, Díaz CJ, Rubio MJ, Ortega R, et al. Radical surgery-peritonectomy and intraoperative intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis in recurrent or primary ovarian cancer. J Surg Oncol. 2006;94(4):316–24.
de Bree E, Romanos J, Michalakis J, Relakis K, Georgoulias V, Melissas J, et al. Intraoperative hyperthermic intraperitoneal chemotherapy with docetaxel as second-line treatment for peritoneal carcinomatosis of gynaecological origin. Anticancer Res. 2003;23(3C):3019–27.
de Bree E, Rosing H, Beijnen JH, Romanos J, Michalakis J, Georgoulias V, et al. Pharmacokinetic study of docetaxel in intraoperative hyperthermic i.P. Chemotherapy for ovarian cancer. Anticancer Drugs. 2003;14(2):103–10.
Kinoshita J, Fushida S, Tsukada T, Oyama K, Watanabe T, Shoji M, et al. Comparative study of the antitumor activity of Nab-paclitaxel and intraperitoneal solvent-based paclitaxel regarding peritoneal metastasis in gastric cancer. Oncol Rep. 2014;32(1):89–96.
Wiernik PH, Schwartz EL, Strauman JJ, Dutcher JP, Lipton RB, Paietta E. Phase I clinical and pharmacokinetic study of taxol. Cancer Res. 1987;47(9):2486–93.
Chen N, Brachmann C, Liu X, Pierce DW, Dey J, Kerwin WS, et al. Albumin-bound nanoparticle (nab) paclitaxel exhibits enhanced paclitaxel tissue distribution and tumor penetration. Cancer Chemother Pharmacol. 2015;76(4):699–712.
Mielke S, Sparreboom A, Mross K. Peripheral neuropathy: a persisting challenge in paclitaxel-based regimes. Eur J Cancer Oxf Engl 1990. 2006;42(1):24–30.
Gelderblom H, Verweij J, van Zomeren DM, Buijs D, Ouwens L, Nooter K, et al. Influence of Cremophor el on the bioavailability of intraperitoneal paclitaxel. Clin Cancer Res Off J Am Assoc Cancer Res. 2002;8(4):1237–41.
Gotloib L, Shostak A. Endocytosis and transcytosis of albumin gold through mice peritoneal mesothelium. Kidney Int. 1995;47(5):1274–84.
Shang D, Peng T, Gou S, Li Y, Wu H, Wang C, et al. High Mobility Group Box Protein 1 Boosts Endothelial Albumin Transcytosis through the RAGE/Src/Caveolin-1 Pathway. Sci Rep. 2016;6:32180.
von Ruhland CJ, Campbell L, Gumbleton M, Jasani B, Newman GR. Immunolocalization of caveolin-1 in rat and human mesothelium. J Histochem Cytochem Off J Histochem Soc. 2004;52(11):1415–25.
ACKNOWLEDGMENTS AND DISCLOSURES
We acknowledge support to ED from Cariplo Foundation for the project (Project 2013–0692). The study was supported in part by funding from Celgene Corporation Grant-ITA-039 (M1266). The authors thank J.D. Baggott for editing the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Procedures involving animals and their care were conducted in conformity with institutional guidelines that comply with national (DL26, March 2014) and international (EEC Council Directive 2010/63, August, 2013) laws and policies and in line with guidelines for the welfare and use of animals in cancer research.
Disclosure of Commercial Interest
None of the authors has any financial and personal relationships with other people or organizations to disclose, that could inappropriately influence their work.
Additional information
Federico Coccolini, Fabio Acocella and Lavinia Morosi equally contributed to this work
Rights and permissions
About this article
Cite this article
Coccolini, F., Acocella, F., Morosi, L. et al. High Penetration of Paclitaxel in Abdominal Wall of Rabbits after Hyperthermic Intraperitoneal Administration of Nab-Paclitaxel Compared to Standard Paclitaxel Formulation. Pharm Res 34, 1180–1186 (2017). https://doi.org/10.1007/s11095-017-2132-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2132-4