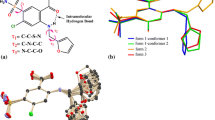
ABSTRACT
Crystal polymorphism of pharmaceuticals has well-known profound effects on the physical, chemical, and pharmaceutical properties of drugs, which can result in changes in the solubility, stability, dissolution, bioavailability, and efficacy of drugs. In this review article, famotidine (FAM), which has a well-known trade name of Pepcid®, was selected as a model drug. Although FAM has three polymorphs (forms A, B and C), forms A and B have been commonly discussed. The active pharmaceutical ingredient (API) in the commercial version of FAM is the metastable form B. FAM has been a concern of FDA because of the physical properties, solubilities, bioavailabilities, or bioequivalencies of the different polymorphic forms. In addition, a patent infringement suit of FAM polymorph had been made sound legal arguments in the pharmaceutical market. We review the solid-state characteristics, thermodynamics, polymorphic transformation, and quality control of FAM in drug products. In particular, pharmaceutical processes, such as grinding, compression, and heating temperature have a significant effect on the polymorphic transformation of FAM. Moreover, environmental humidity and residual water content should be well controlled to prevent polymorphic transformation of FAM during pharmaceutical processing. Several thermal and spectroscopic analytical techniques used for qualitative and quantitative determinations of polymorphic transformation of FAM after different treatments or quality control of FAM in the commercial tablets before and after the expiration dates have been discussed.








Similar content being viewed by others
REFERENCES
Purohit R, Venugopalan P. Polymorphism: an overview. Resonance. 2009;14:882–93.
Brittain HG. Polymorphism in pharmaceutical solids. 2nd ed. New York: Informa HealthCare; 2009.
Byrn S, Pfeiffer R, Ganey M, Hoiberg C, Poochikian G. Pharmaceutical solids: a strategic approach to regulatory considerations. Pharm Res. 1995;12:945–54.
Lee AY, Erdemir D, Myerson AS. Crystal polymorphism in chemical process development. Annu Rev Chem Biomol Eng. 2011;2:259–80.
Karpinski PH. Polymorphism of active pharmaceutical ingredients. Chem Eng Technol. 2006;29:233–7.
Chawla G, Bansal AK. Challenges in polymorphism of pharmaceuticals. CRIPS. 2004;5:9–12.
Newman W, Byrn S. Solid-state analysis of the active pharmaceutical ingredient in drug products. Drug Discov Today. 2003;8:898–905.
Raw AS, Furness MS, Gill DS, Adams Jr RC, Holcombe FO, Yu LX. Regulatory considerations of pharmaceutical solid polymorphism in Abbreviated New Drug Applications (ANDAs). Adv Drug Deliv Rev. 2004;56:397–414.
Llinàs A, Goodman JM. Polymorph control: past, present and future. Drug Discov Today. 2008;13:198–210.
Morissette SL, Soukasene S, Levinson D, Cima MJ, Almarsson O. Elucidation of crystal form diversity of the HIV protease inhibitor ritonavir by high-throughput crystallization. Proc Natl Acad Sci U S A. 2003;100:2180–4.
Center for Drug Evaluation and Research. Guidance for industry; ANDAs: pharmaceutical solid polymorphism: chemistry, manufacturing, and controls information. Rockville: US FDA; 2007.
EMEA ICH Q6A Guideline. Specifications: test procedures and acceptance criteria for new drug substances and new drug products; 2000.
Payghan SA, Patwekar S, Kate VK, Khavane K, Purohit S. Pharmaceutical solid polymorphism: approach in regulatory consideration. J Glob Pharma Technol. 2010;2(1):8–16.
WHO. Guidelines on submission of documentation for a multisource (generic) finished pharmaceutical product for the WHO Prequalification of Medicines Programme: quality part. WHO Tech Rep Ser. 2012;970:122–96.
Chaudhary A, Nagaich U, Gulati N, Sharma VK, Khosa RL. Enhancement of solubilization and bioavailability of poorly soluble drugs by physical and chemical modifications: a recent review. J Adv Pharm Educ Res. 2012;2(1):32–67.
Pandey B, Lohray VB, Lohray BB. Importance of polymorphs and salts in the pharmaceutical industry. In: Chorghade MS, editor. Drug discovery and development: drug development, vol. 2. New Jersey: Wiley; 2007. p. 201–18.
Lu J, Rohani S. Polymorphism and crystallization of active pharmaceutical ingredients (APIs). Curr Med Chem. 2009;16:884–905.
Morissette SL, Almarsson O, Peterson ML, Remenar JF, Read MJ, Lemmo AV, et al. High-throughput crystallization: polymorphs, salts, co-crystals and solvates of pharmaceutical solids. Adv Drug Deliv Rev. 2004;56:275–300.
Rodríguez-Spong B, Price CP, Jayasankar A, Matzger AJ, Rodríguez-Hornedo N. General principles of pharmaceutical solid polymorphism: a supramolecular perspective. Adv Drug Deliv Rev. 2004;56:241–74.
Zhang GZ, Law D, Schmitt EA, Qiu Y. Phase transformation considerations during process development and manufacture of solid oral dosage forms. Adv Drug Deliv Rev. 2004;56:371–90.
Bianco S, Caron V, Tajber L, Corrigan OI, Nolan L, Hu Y, et al. Modification of the solid-state nature of sulfathiazole and sulfathiazole sodium by spray drying. AAPS PharmSciTech. 2012;13:647–60.
Huttenrauch R, Fricke S, Zielke P. Mechanical activation of pharmaceutical systems. Pharm Res. 1985;2:253–7.
Otsuka M, Otsuka K, Kaneniwa N. Relation between polymorphic transformation pathway during grinding and the physicochemical properties of bulk powders for pharmaceutical preparations. Drug Dev Ind Pharm. 1994;20:1649–60.
Shakhtshneider TP. Phase transformations and stabilization of metastable states of molecular crystals under mechanical activation. Solid State Ionics. 1997;101–103:851–6.
Hédoux A, Guinet Y, Paccou L, Danède F, Derollez P. Polymorphic transformation of anhydrous caffeine upon grinding and hydrostatic pressurizing analyzed by low-frequency raman spectroscopy. J Pharm Sci. 2013;102:162–70.
Guo Z, Ma M, Wang T, Chang D, Jiang T, Wang S. A kinetic study of the polymorphic transformation of nimodipine and indomethacin during high shear granulation. AAPS PharmSciTech. 2011;12:610–9.
Chan HK, Doelker E. Polymorphic transformation of some drugs under compression. Drug Dev Ind Pharm. 1985;11:315–32.
Willart JF, Descamps M. Solid state amorphization of pharmaceuticals. Mol Pharm. 2008;5:905–20.
Yu L. Amorphous pharmaceutical solids: preparation, characterization and stabilization. Adv Drug Deliv Rev. 2001;48:27–42.
Reutzel-Edens SM. Achieving polymorph selectivity in the crystallization of pharmaceutical solids: basic considerations and recent advances. Curr Opin Drug Discov Dev. 2006;9:806–15.
Erdemir D, Lee AY, Myerson AS. Polymorph selection: the role of nucleation, crystal growth and molecular modeling. Curr Opin Drug Discov Dev. 2007;10:746–55.
Yu LX, Lionberger RA, Raw AS, D’Costa R, Wu H, Hussain AS. Applications of process analytical technology to crystallization processes. Adv Drug Deliv Rev. 2004;56:349–69.
Nagy ZK, Braatz RD. Advances and new directions in crystallization control. Annu Rev Chem Biomol Eng. 2012;3:55–75.
Storey RA, Ymén I. Solid state characterization of pharmaceuticals. West Sussex: Wiley; 2011.
Bauer J, Spanton S, Henry R, Quick J, Dziki W, Porter W, et al. Ritonavir: an extraordinary example of conformational polymorphism. Pharm Res. 2001;18:859–66.
Dammann HG. Clinical efficacy of famotidine in the treatment of acid-related diseases: an overview. Hepato-Gastroenterology. 1990;37 Suppl 1:2–5.
Langtry HD, Grant SM, Goa KL. Famotidine. An updated review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in peptic ulcer disease and other allied diseases. Drugs. 1989;38:551–90.
Schiff M, Peura D. HZT-501 (DUEXIS(®); ibuprofen 800 mg/famotidine 26.6 mg) gastrointestinal protection in the treatment of the signs and symptoms of rheumatoid arthritis and osteoarthritis. Exp Rev Gastroenterol Hepatol. 2012;6:25–35.
Yamamoto K, Hojo H, Koshima I, Chung UI, Ohba S. Famotidine suppresses osteogenic differentiation of tendon cells in vitro and pathological calcification of tendon in vivo. J Orthop Res. 2012;30:1958–62.
Fogg TB, Semple D. Combination therapy with H2 and H1 antihistamines in acute, non-compromising allergic reactions. Emerg Med J. 2008;25:165–6.
Molinari SP, Kaminski R, Rocco AD, Yahr MD. The use of famotidine in the treatment of Parkinson’s disease: a pilot study. J Neural Transm Park Dis Dement Sect. 1995;9:243–7.
Breitner JCS, Welsh KA, Helms MJ, Gaskell PC, Gau BA, Roses AD, et al. Delayed onset of Alzheimer’s disease with nonsteroidal anti-inflammatory and histamine H2 blocking drugs. Neurobiol Aging. 1995;16:523–30.
Fahmy RH, Kassem MA. Enhancement of famotidine dissolution rate through liquisolid tablets formulation: in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2008;69:993–1003.
Patel DJ, Patel JK, Pandya VM. Improvement in the dissolution of poorly water soluble drug using media milling technique. Thai J Pharm Sci. 2010;34:155–64.
Singhal D, Curatolo W. Drug polymorphism and dosage form design: a practical perspective. Adv Drug Deliv Rev. 2004;56:335–47.
Lu J, Wang XJ, Yang X, Ching CB. Characterization and selective crystallization of famotidine polymorphs. J Pharm Sci. 2007;96:2457–68.
Hassan MA, Salem MS, Sueliman MS, Najib NM. Characterization of famotidine polymorphic forms. Int J Pharm. 1997;149:227–32.
Német Z, Kis GC, Pokol G, Demeter A. Quantitative determination of famotidine polymorphs: X-ray powder diffractometric and Raman spectrometric study. J Pharm Biomed Anal. 2009;49:338–46.
Lin SY, Cheng WT, Wang SL. Thermodynamic and kinetic characterization of polymorphic transformation of famotidine during grinding. Int J Pharm. 2006;318:86–91.
Chieng N, Rades T, Aaltonen J. An overview of recent studies on the analysis of pharmaceutical polymorphs. J Pharm Biomed Anal. 2011;55:618–44.
Hegedüs B, Bod P, Harsányi K, Péter I, Kálmán A, Párkányi L. Comparison of the polymorphic modifications of famotidine. J Pharm Biomed Anal. 1989;7:563–9.
Sagdinc S, Bayari S. Experimental and theoretical infrared spectra of famotidine and its interaction with ofloxacin. J Mol Struct. 2005;744–747:369–76.
Overgaard J, Hibbs DE. The experimental electron density in polymorphs A and B of the anti-ulcer drug famotidine. Acta Crystallogr A. 2004;60:480–7.
Cheng WT, Lin SY, Li MJ. Raman microspectroscopic mapping or thermal system used to investigate milling-induced solid-state conversion of famotidine polymorphs. J Raman Spectrosc. 2007;38:1595–601.
Cheng WT, Lin SY. Famotidine polymorphic transformation in the grinding process significantly depends on environmental humidity or water content. Int J Pharm. 2008;357:164–8.
Beckmann W. Seeding the desired polymorph: background, possibilities, limitations, and case studies. Org Process Res Dev. 2000;4:372–83.
Lu J, Wang XJ, Yang X, Ching CB. Polymorphism and crystallization of famotidine. Cryst Growth Des. 2007;7:1590–8.
Debnath S, Suryanarayanan R. Influence of processing-induced phase transformations on the dissolution of theophylline tablets. AAPS PharmSciTech. 2004;5:39–49.
Govindarajan R, Suryanarayanan R. Processing-induced phase transformations and their implications on pharmaceutical product quality. In: Hilfiker R, editor. Polymorphism: in the pharmaceutical industry. Weiheim: Wiley-VCH Verlag GmbH & Co.; 2006. p. 333–64.
Fernandez-Bertran JF. Mechanochemistry—an overview. Pure Appl Chem. 1999;71:581–6.
Colombo I, Grassi G, Grassi M. Drug mechanochemical activation. J Pharm Sci. 2009;98:3961–86.
Altheimer BD, Pagola S, Zeller M, Mehta MA. Mechanochemical conversions between crystalline polymorphs of a complex organic solid. Cryst Growth Des. 2013;13:3447–53.
Ferenczy GG, Párkányi L, Ángyán JG, Kálmán A, Hegedűs B. Crystal and electronic structure of two polymorphic modifi cations of famotidine. An experimental and theoretical study. J Mol Struct THEOCHEM. 2000;503:73–9.
Német Z, Sajó I, Demeter A. Rietveld refinement in the routine quantitative analysis of famotidine polymorphs. J Pharm Biomed Anal. 2010;51:572–6.
Nagaraju R, Prathusha AP, Subhash Chandra Bose P, Kaza R, Bharathi K. Preparation and evaluation of famotidine polymorphs. Curr Drug Discov Technol. 2010;7:106–16.
Cheng WT, Lin SY, Wang SL. Differential scanning calorimetry with curve-fitting program used to quantitatively analyze the polymorphic transformation of famotidine in the compressed compact. Drug Dev Ind Pharm. 2008;34:1368–75.
Roux MV, Dávalos JZ, Jiménez P. Effect of pressure on the polymorphic forms of famotidine. Thermochim Acta. 2002;394:19–24.
Német Z, Hegedűs B, Szántay Jr C, Sztatisz J, Pokol G. Pressurization effects on the polymorphic forms of famotidine. Thermochim Acta. 2005;430:35–41.
Lin SY, Cheng WT, Wang SL. Thermal micro-Raman spectroscopic study of polymorphic transformation of famotidine under different compression pressures. J Raman Spectrosc. 2007;38:39–43.
Auer ME, Griesser UJ, Sawatzki J. Qualitative and quantitative study of polymorphic forms in drug formulations by near infrared FT-Raman spectroscopy. J Mol Struct. 2003;661–662:307–17.
Nishizawa S, Takeda MW, Tani M. Terahertz time-domain spectroscopy (THz-TDS) approach to the quality control on pharmaceutical products. Proc 3rd Int Work Far Infrared Technol. 2010;16a-5:106–11.
Ajito K, Ueno Y, Song HJ, Tamechika E, Kukutsu N. Terahertz spectroscopic imaging of polymorphic forms in pharmaceutical crystals. Mol Cryst Liq Cryst. 2011;538:33–8.
Brettmann BK, Cheng K, Myerson AS, Trout BL. Electrospun formulations containing crystalline active pharmaceutical ingredients. Pharm Res. 2013;30:238–46.
Wildfong PL, Hancock BC, Moore MD, Morris KR. Towards an understanding of the structurally based potential for mechanically activated disordering of small molecule organic crystals. J Pharm Sci. 2006;95:2645–56.
Rasenack N, Muller BW. Micron-size drug particles: common and novel micronization techniques. Pharm Dev Technol. 2004;9:1–13.
Vippagunta SR, Brittain HG, Grant DJ. Crystalline solids. Adv Drug Deliv Rev. 2001;48:3–26.
Patel S, Kaushal AM, Bansal AK. Compression physics in the formulation development of tablets. Crit Rev Ther Drug Carrier Syst. 2006;23:1–65.
Ajito K, Ueno Y, Song HJ, Tamechika E, Kukutsu N. Terahertz chemical imaging of molecular networks for pharmaceutical applications. ECS Trans. 2011;35(7):57–165.
Ajito K, Ueno Y, Song HJ. Visualization of pharmaceutical drug molecules by terahertz chemical imaging. NTT Tech Rev. 2012;10(2):1–6.
Ajito K, Ueno Y. THz chemical imaging for biological applications. IEEE Trans Terahertz Sci Technol. 2011;1:293–300.
Kawase M, Saito T, Ogawa M, Uejima H, Hatsuda Y, Kawanishi S, et al. Application of terahertz absorption spectroscopy to evaluation of aging variation of medicine. Anal Sci. 2011;27:209–12.
Kovac C. Drug company takes 10 others to court for patent infringement. BMJ. 2001;323:252.
Takenaka T. Patent infringement damages in Japan and the United States: will increased patent infringement damage awards revive the Japanese economy? Wash Univ J Law Policy. 2002;2:309–70.
Hussey DT. Comparing notes: selected current issues in drug patent listing and litigation in Canada and the United States. Can Intellect Prop Law Rev. 2003;19:7–94.
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by National Science Council, Taipei, Taiwan, Republic of China (NSC-95-2320-B-075-002-MY2). The authors also thank Misses W. T. Cheng, M. J. Li and Dr. S. L. Wang for their contributions on these studies and Miss Y. T. Huang for figures drawing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, SY. An Overview of Famotidine Polymorphs: Solid-State Characteristics, Thermodynamics, Polymorphic Transformation and Quality Control. Pharm Res 31, 1619–1631 (2014). https://doi.org/10.1007/s11095-014-1323-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1323-5