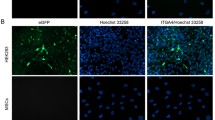
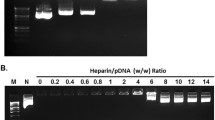
Abstract
Purpose
Neurons in post-traumatized mammalian central nervous system show only limited degree of regeneration, which can be attributed to the presence of neurite outgrowth inhibitors in damaged myelin and glial scar, and to the apoptosis of severed central neurons and glial cells during secondary Wallerian degeneration. RhoA GTPase has been implicated as the common denominator in these counter-regeneration events, which shows significant and persistent up-regulation for weeks in injured spinal cord and cerebral infarct after stroke. While the exoenzyme C3 transferase is a potent RhoA inhibitor, its extremely low efficiency of cell entry and degradation in vivo has restricted the therapeutic value. This study aims to circumvent these problems by developing a membrane-permeating form of C3 transferase and a biopolymer-based microsphere depot system for sustainable controlled release of the protein.
Materials and Methods
A membrane-permeating form of C3 transferase was developed by fusing a Tat (trans-activating transcription factor) transduction domain of human immunodeficiency virus to its amino terminal using standard molecular cloning techniques. After confirming efficient cell entry into epithelial and neuroblastoma cells, the resulting recombinant protein TAT-C3 was encapsulated in biocompatible polymer poly(d,l-lactide-co-glycolide) in the form of microspheres by a water-in-oil-in-water (W/O/W) emulsion method. By blending capped and uncapped form of the polymer at different ratios, TAT-C3 protein release profile was modified to suit the expression pattern of endogenous RhoA during CNS injuries. Bioactivity of TAT-C3 released from microspheres was assessed by RhoA ribosylation assay.
Results
In contrast to wild-type C3 transferase, the modified TAT-C3 protein was found to efficiently enter NIH3T3 and N1E-115 neuroblastoma cells as early as 6 hours of incubation. The fusion of TAT sequence to C3 transferase imposed no appreciable effects on its biological activity in promoting neurite outgrowth through RhoA inhibition. Characterization of TAT-C3 encapsulation in various blends of capped/uncapped PLGA polymer revealed the 30:70 formulation to be optimal in attaining a mild initial burst release of 25%, followed by a subsequent average daily release of 2.3% of encapsulated protein over one month, matching the change in RhoA level in severed brain and spinal cord. Importantly, TAT-C3 released from the microspheres remained active up to the first three weeks of incubation.
Conclusion
Enhanced cell entry of TAT-C3 circumvents the need to administer high dose of the protein to site of injury. The encapsulation of TAT-C3 in different blends of capped/uncapped PLGA microspheres allows adjustment of protein release profile to suit the pattern of RhoA expression in injured CNS.









Similar content being viewed by others
References
E. Emery, P. Aldana, M. B. Bunge, W. Puckett, A. Srinivasan, R. W. Keane, J. Bethea, and A. D. Levi. Apoptosis after traumatic human spinal cord injury. J. Neurosurg. 89:911–920 (1998).
M. J. Crowe, J. C. Bresnahan, S. L. Shuman, J. N. Masters, and M. S. Beattie. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat. Med. 3:73–76 (1997).
S. Casha, W. R. Yu, and M. G. Fehlings. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neuroscience 103:203–218 (2001).
S. Ramon y Cajal. Degeneration and regeneration of the nervous system. Oxford University Press, London, 1928.
M. Berry. Post-injury myelin-breakdown products inhibit axonal growth: a hypothesis to explain the failure of axonal regeneration in the mammalian central nervous system. Bibl. Anat. 23:1–11 (1982).
P. Caroni and M. E. Schwab. Two membrane protein fractions from rat central myelin with inhibitory properties for neurite growth and fibroblast spreading. J. Cell Biol. 106:1281–1288 (1988).
M. Domeniconi and M. T. Filbin. Overcoming inhibitors in myelin to promote axonal regeneration. J. Neurol. Sci. 233:43–47 (2005).
K. C. Wang, J. A. Kim, R. Sivasankaran, R. Segal, and Z. He. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 420:74–78 (2002).
F. J. Liuzzi and R. J. Lasek. Astrocytes block axonal regeneration in mammals by activating the physiological stop pathway. Science 237:642–645 (1987).
P. J. Reier, L. J. Stensaas, and L. Guth. The astrocytic scar as an impediment to regeneration in the central nervous system. In C. C. Kao, R. P. Bunge, and P. J. Reier (eds.), Spinal cord reconstruction, Raven, New York, 1983, pp. 163–195.
R. A. Asher, D. A. Morgenstern, P. S. Fidler, K. H. Adcock, A. Oohira, J. E. Braistead, J. M. Levine, R. U. Margolis, J. H. Rogers, and J. W. Fawcett. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J. Neurosci. 20:2427–2438 (2000).
R. A. Asher, D. A. Morgenstern, M. C. Shearer, K. H. Adcock, P. Pesheva, and J. W. Fawcett. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J. Neurosci. 22:2225–2236 (2002).
M. T. Fitch and J. Silver. Activated macrophages and the blood–brain barrier: inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp. Neurol. 148:587–603 (1997).
R. A. Asher, D. A. Morgenstern, L. D. Moon, and J. W. Fawcett. Chondroitin sulphate proteoglycans: inhibitory components of the glial scar. Prog. Brain Res. 132:611–619 (2001).
D. A. Morgenstern, R. A. Asher, and J. W. Fawcett. Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain Res. 137:313–332 (2002).
Y. Zhang, J. K. Winterbottom, M. Schachner, A. R. Lieberman, and P. N. Anderson. Tenascin-C expression and axonal sprouting following injury to the spinal dorsal columns in the adult rat. J. Neurosci. Res. 49:433–450 (1997).
F. De Winter, M. Oudega, A. J. Lankhorst, F. P. Hamers, B. Blits, M. J. Ruitenberg, R. J. Pasterkamp, W. H. Gispen, and J. Verhaagen. Injury-induced class 3 semaphorin expression in the rat spinal cord. Exp. Neurol. 175:61–75 (2002).
R. J. Pasterkamp, P. N. Anderson, and J. Verhaagen. Peripheral nerve injury fails to induce growth of lesioned ascending dorsal column axons into spinal cord scar tissue expressing the axon repellent Semaphorin3A. Eur. J. Neurosci. 13:457–471 (2001).
C. Moreau-Fauvarque, A. Kumanogoh, E. Camand, C. Jaillard, G. Barbin, I. Boquet, C. Love, E. Y. Jones, H. Kikutani, C. Lubetzki, I. Dusart, and A. Chedotal. The transmembrane semaphorin Sema4D/CD100, an inhibitor of axonal growth, is expressed on oligodendrocytes and upregulated after CNS lesion. J. Neurosci. 23:9229–9239 (2003).
P. P. Monnier, A. Sierra, J. M. Schwab, S. Henke-Fahle, and B. K. Mueller. The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol. Cell. Neurosci. 22:319–330 (2003).
V. Perrot, J. Vazquez-Prado, and J. S. Gutkind. Plexin B regulates Rho through the guanine nucleotide exchange factors leukemia-associated Rho GEF (LARG) and PDZ-RhoGEF. J. Biol. Chem. 277:43115–43120 (2002).
J. M. Swiercz, R. Kuner, J. Behrens, and S. Offermanns. Plexin-B1 directly interacts with PDZ-RhoGEF/LARG to regulate RhoA and growth cone morphology. Neuron 35:51–63 (2002).
E. J. Rubin, D. M. Gill, P. Boquet, and M. R. Popoff. Functional modification of a 21-kilodalton G protein when ADP-ribosylated by exoenzyme C3 of Clostridium botulinum. Mol. Cell Biol. 8:418–426 (1988).
P. Dergham, B. Ellezam, C. Essagian, H. Avedissian, W. D. Lubell, and L. McKerracher. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci. 22:6570–6577 (2002).
A. E. Fournier, B. T. Takizawa, and S. M. Strittmatter. Rho kinase inhibition enhances axonal regeneration in the injured CNS. J. Neurosci. 23:1416–1423 (2003).
Z. Jin and S. M. Strittmatter. Rac1 mediates collapsin-1-induced growth cone collapse. J. Neurosci. 17:6256–6263 (1997).
M. Lehmann, A. Fournier, I. Selles-Navarro, P. Dergham, A. Sebok, N. Leclerc, G. Tigyi, and L. McKerracher. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J. Neurosci. 19:7537–7547 (1999).
B. Niederost, T. Oertle, J. Fritsche, R. A. McKinney, and C. E. Bandtlow. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. 22:10368–10376 (2002).
A. D. Frankel and C. O. Pabo. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55:1189–1193 (1988).
M. Green and P. M. Loewenstein. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 55:1179–1188 (1988).
S. Fawell, J. Seery, Y. Daikh, C. Moore, L. L. Chen, B. Pepinsky, and J. Barsoum. Tat-mediated delivery of heterologous proteins into cells. Proc. Natl. Acad. Sci. U. S. A 91:664–668 (1994).
S. Conrad, H. J. Schluesener, K. Trautmann, N. Joannin, R. Meyermann, and J. M. Schwab. Prolonged lesional expression of RhoA and RhoB following spinal cord injury. J. Comp. Neurol. 487:166–175 (2005).
C. Brabeck, M. Mittelbronn, K. Bekure, R. Meyermann, H. J. Schluesener, and J. M. Schwab. Effect of focal cerebral infarctions on lesional RhoA and RhoB expression. Arch. Neurol. 60:1245–1249 (2003).
J. P. Benoit, N. Faisant, M. C. Venier-Julienne, and P. Menei. Development of microspheres for neurological disorders: from basics to clinical applications. J. Control. Release 65:285–296 (2000).
D. F. Emerich, M. A. Tracy, K. L. Ward, M. Figueiredo, R. Qian, C. Henschel, and R. T. Bartus. Biocompatibility of poly (DL-lactide-co-glycolide) microspheres implanted into the brain. Cell Transplant. 8:47–58 (1999).
P. Menei, V. Daniel, C. Montero-Menei, M. Brouillard, A. Pouplard-Barthelaix, and J. P. Benoit. Biodegradation and brain tissue reaction to poly(D,L-lactide-co-glycolide) microspheres. Biomaterials 14:470–478 (1993).
K. L. Guan and J. E. Dixon. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal. Biochem. 192:262–267 (1991).
J. M. Pean, F. Boury, M. C. Venier-Julienne, P. Menei, J. E. Proust, and J. P. Benoit. Why does PEG 400 co-encapsulation improve NGF stability and release from PLGA biodegradable microspheres? Pharm. Res. 16:1294–1299 (1999).
C. Yan, J. H. Resau, M. West, W. L. Rill, and M. Kende. Characterization and morphological analysis of protein-loaded poly(lactide-co-glycolide) microparticles prepared by water-in-oil-in-water emulsion technique. J. Control. Release 32:231–241 (1994).
E. P. Magre and A. P. Sam. Hydrolytic degradation of PLAGA, calculation of rate constants from various types of in-vitro degradation curves. J. Control. Release 48:318–320 (1997).
S. T. Dillon and L. A. Feig. Purification and assay of recombinant C3 transferase. Methods Enzymol. 256:174–184 (1995).
R. Kozma, S. Sarner, S. Ahmed, and L. Lim. Rho family GTPases and neuronal growth cone remodelling: relationship between increased complexity induced by Cdc42Hs, Rac1, and acetylcholine and collapse induced by RhoA and lysophosphatidic acid. Mol. Cell Biol. 17:1201–1211 (1997).
Y. Kloog, J. Axelrod, and I. Spector. Protein carboxyl methylation increases in parallel with differentiation of neuroblastoma cells. J. Neurochem. 40:522–529 (1983).
H. Li, H. C. Chen, and F. L. Huang. Identification of a rapidly dephosphorylating 95-kDa protein as elongation factor 2 during 8-Br-cAMP treatment of N1E115 neuroblastoma cells. Biochem. Biophys. Res. Commun. 217:131–137 (1995).
T. Ishii, E. Satoh, and M. Nishimura. Integrin-linked kinase controls neurite outgrowth in N1E-115 neuroblastoma cells. J. Biol. Chem. 276:42994–43003 (2001).
J. Yamauchi, Y. Miyamoto, A. Sanbe, and A. Tanoue. JNK phosphorylation of paxillin, acting through the Rac1 and Cdc42 signaling cascade, mediates neurite extension in N1E-115 cells. Exp. Cell Res. 312:2954–2961 (2006).
C. G. Pitt, M. M. Gratzl, G. L. Kimmel, J. Surles, and A. Schindler. Aliphatic polyesters II. The degradation of poly (DL-lactide), poly (epsilon-caprolactone), and their copolymers in vivo. Biomaterials 2:215–220 (1981).
N. Faisant, J. Siepmann, and J. P. Benoit. PLGA-based microparticles: elucidation of mechanisms and a new, simple mathematical model quantifying drug release. Eur. J. Pharm. Sci. 15:355–366 (2002).
J. Wang, B. M. Wang, and S. P. Schwendeman. Characterization of the initial burst release of a model peptide from poly(D,L-lactide-co-glycolide) microspheres. J. Control. Release 82:289–307 (2002).
J. M. Pean, M. C. Venier-Julienne, F. Boury, P. Menei, B. Denizot, and J. P. Benoit. NGF release from poly(D,L-lactide-co-glycolide) microspheres. Effect of some formulation parameters on encapsulated NGF stability. J. Control. Release 56:175–187 (1998).
C. Sturesson and J. Carlfors. Incorporation of protein in PLG-microspheres with retention of bioactivity. J. Control. Release 67:171–178 (2000).
W. Friess and M. Schlapp. Modifying the release of gentamicin from microparticles using a PLGA blend. Pharm. Dev. Technol. 7:235–248 (2002).
M. A. Tracy, K. L. Ward, L. Firouzabadian, Y. Wang, N. Dong, R. Qian, and Y. Zhang. Factors affecting the degradation rate of poly(lactide-co-glycolide) microspheres in vivo and in vitro. Biomaterials 20:1057–1062 (1999).
W. I. Li, K. W. Anderson, R. C. Mehta, and P. P. Deluca. Prediction of solvent removal profile and effect on properties for peptide-loaded PLGA microspheres prepared by solvent extraction/ evaporation method. J. Control. Release 37:199–214 (1995).
J. L. Cleland and A. J. Jones. Stable formulations of recombinant human growth hormone and interferon-gamma for microencapsulation in biodegradable microspheres. Pharm. Res. 13:1464–1475 (1996).
M. Wolf, M. Wirth, F. Pittner, and F. Gabor. Stabilisation and determination of the biological activity of L-asparaginase in poly(D,L-lactide-co-glycolide) nanospheres. Int. J. Pharm. 256:141–152 (2003).
M. Morlock, H. Koll, G. Winter, and T. Kissel. Microencapsulation of rh-erythropoietin, using biodegradable poly(,-lactide-co-glycolide): protein stability and the effects of stabilizing excipients. Eur. J. Pharm. Biopharm. 43:29–36 (1997).
H. Sah. Stabilization of proteins against methylene chloride/water interface-induced denaturation and aggregation. J. Control. Release 58:143–151 (1999).
C. Perez-Rodriguez, N. Montano, K. Gonzalez, and K. Griebenow. Stabilization of alpha-chymotrypsin at the CH2Cl2/water interface and upon water-in-oil-in-water encapsulation in PLGA microspheres. J. Control. Release. 89:71–85 (2003).
E. Sahai and M. F. Olson. Purification of TAT-C3 Exoenzyme. In W. E. Balch, C. J. Der, and A. Hall (eds.), Methods in Enzymology—Regulators and Effectors of Small GTPases: Rho Family, Academic Press, 2006, pp. 128–140.
J. Park, J. S. Kim, K. C. Jung, H. J. Lee, J. I. Kim, J. Kim, J. Y. Lee, J. B. Park, and S. Y. Choi. Exoenzyme Tat-C3 inhibits association of zymosan particles, phagocytosis, adhesion, and complement binding in macrophage cells. Mol. Cells 16:216–223 (2003).
V. Sauzeau, E. Le Mellionnec, J. Bertoglio, E. Scalbert, P. Pacaud, and G. Loirand. Human urotensin II-induced contraction and arterial smooth muscle cell proliferation are mediated by RhoA and Rho-kinase. Circ. Res. 88:1102–1104 (2001).
G. Gadea, Y. Boublik, S. Delga, and P. Roux. Efficient production of Clostridium botulinum exotoxin C3 in bacteria: a screening method to optimize production yields. Protein Expr. Purif. 40:164–168 (2005).
X. Z. Liu, X. M. Xu, R. Hu, C. Du, S. X. Zhang, J. W. McDonald, H. X. Dong, Y. J. Wu, G. S. Fan, M. F. Jacquin, C. Y. Hsu, and D. W. Choi. Neuronal and glial apoptosis after traumatic spinal cord injury. J. Neurosci. 17:5395–5406 (1997).
S. L. Shuman, J. C. Bresnahan, and M. S. Beattie. Apoptosis of microglia and oligodendrocytes after spinal cord contusion in rats. J. Neurosci. Res. 50:798–808 (1997).
S. D. Grossman, L. J. Rosenberg, and J. R. Wrathall. Temporal–spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp. Neurol. 168:273–282 (2001).
C. I. Dubreuil, M. J. Winton, and L. McKerracher. Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. J. Cell Biol. 162:233–243 (2003).
I. J. Castellanos, R. Crespo, and K. Griebenow. Poly(ethylene glycol) as stabilizer and emulsifying agent: a novel stabilization approach preventing aggregation and inactivation of proteins upon encapsulation in bioerodible polyester microspheres. J. Control. Release 88:135–145 (2003).
A. Aubert-Pouessel, M. C. Venier-Julienne, A. Clavreul, M. Sergent, C. Jollivet, C. N. Montero-Menei, E. Garcion, D. C. Bibby, P. Menei, and J. P. Benoit. In vitro study of GDNF release from biodegradable PLGA microspheres. J. Control. Release 95:463–475 (2004).
S. P. Schwendeman, M. Cardamone, M. R. Brandon, A. Kilbanov, and R. Langer. Stability of proteins and their delivery from biodegradable polymer microspheres. In Microparticulate systems for the delivery of proteins and vaccines, Marcel Dekker, New York, 1996.
J. Yang and J. L. Cleland. Factors affecting the in vitro release of recombinant human interferon-gamma (rhIFN-gamma) from PLGA microspheres. J. Pharm. Sci. 86:908–914 (1997).
A. P. Nicholas, C. McInnis, K. B. Gupta, W. W. Snow, D. F. Love, D. W. Mason, T. M. Ferrell, J. K. Staas, and T. R. Tice. The fate of biodegradable microspheres injected into rat brain. Neurosci. Lett. 323:85–88 (2002).
T. G. Park, W. Lu, and G. Crotts. Importance of in vitro experimental conditions on protein release kinetics, stability and polymer degradation in protein encapsulated poly (-lactic acid-co-glycolic acid) microspheres. J. Control. Release 33:211–222 (1995).
S. C. Piscitelli, W. G. Reiss, W. D. Figg, and W. P. Petros. Pharmacokinetic studies with recombinant cytokines. Scientific issues and practical considerations. Clin. Pharmacokinet. 32:368–381 (1997).
A. Sanchez, M. Tobio, L. Gonzalez, A. Fabra, and M. J. Alonso. Biodegradable micro- and nanoparticles as long-term delivery vehicles for interferon-alpha. Eur. J. Pharm. Sci. 18:221–229 (2003).
L. Chen, R. N. Apte, and S. Cohen. Characterization of PLGA microspheres for the controlled delivery of IL-1[alpha] for tumor immunotherapy. J. Control. Release 43:261–272 (1997).
R. Gref, Y. Minamitake, M. T. Peracchia, V. Trubetskoy, V. Torchilin, and R. Langer. Biodegradable long-circulating polymeric nanospheres. Science 263:1600–1603 (1994).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tan, E.Y.M., Law, J.W.S., Wang, CH. et al. Development of a Cell Transducible RhoA Inhibitor TAT-C3 Transferase and its Encapsulation in Biocompatible Microspheres to Promote Survival and Enhance Regeneration of Severed Neurons. Pharm Res 24, 2297–2308 (2007). https://doi.org/10.1007/s11095-007-9454-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9454-6