Abstract
Purpose
The development and validation of a physiology-based absorption model for orally administered drugs in monkeys is described.
Materials and Methods
Physiological parameters affecting intestinal transit and absorption of an orally administered drug in monkeys have been collected from the literature and implemented in a physiological model for passive absorption previously developed for rats and humans. Predicted fractions of dose absorbed have been compared to experimentally observed values for a set of N = 37 chemically diverse drugs. A sensitivity analysis was performed to assess the influence of various physiological model parameters on the predicted fraction dose absorbed.
Results
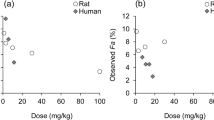
A Pearson’s correlation coefficient of 0.94 (95% confidence interval: [0.88, 0.97]; p < 0.0001) between the predicted and observed fraction dose absorbed in monkeys was obtained for compounds undergoing non-solubility limited passive absorption (N = 29). The sensitivity analysis revealed that the predictions of fractions dose absorbed in monkeys are very sensitive with respect to inter-individual variations of the small intestinal transit time.
Conclusions
The model is well suited to predict the fraction dose absorbed of passively absorbed compounds after oral administration and to assess the influence of inter-individual physiological variability on oral absorption in monkeys.



Similar content being viewed by others
References
K. Ikegami, K. Tagawa, S. Narisawa, and T.Osawa. Suitability of the cynomolgus monkey as an animal model for drug absorption studies of oral dosage forms from the viewpoint of gastrointestinal physiology. Biol. Pharm. Bull. 26:1442–1447 (2003).
W. L. Chiou, and P. W. Buehler. Comparison of oral absorption and bioavailablity of drugs between monkey and human. Pharm. Res. 19:868–874 (2002).
L. L. de Zwart, C. J. M. Rompelberg, A. J. A. M. Sips, J. Welink, and J. G. M. van Engelen. Anatomical and physiological differences between various species used in studies on the pharmacokinetics and toxicology of xenobiotics. A review of the literature. 1999. Report No.: National Institute of Public Health and the Environment. RIVM report 623860 010.
J. B. Dressman and K. Yamada. Animal models for oral drug absorption. In N. Y. N. Dekker (ed.), Drugs Pharm. Sci. 48:235–266 (1991).
T. T. Kararli. Comparison of the gastrointestinal anatomy, physiology, and, biochemistry of humans and commonly used laboratory animals. Biopharm. Drug Dispos. 16:351–380 (1995).
S. Willmann, W. Schmitt, J. Keldenich, and J. B. Dressman. A physiologic model for simulating gastrointestinal flow and drug absorption in rats. Pharm. Res. 20:1766–1771 (2003).
S. Willmann, W. Schmitt, J. Keldenich, J. Lippert, and J. B. Dressman. A physiological model for the estimation of the fraction dose absorbed in humans. J. Med. Chem. 47:4022–4031 (2004).
S. Willmann, J. Lippert, and W. Schmitt. From physicochemistry to absorption and distribution: predictive mechanistic modelling and computational tools. Expert Opin. Drug Meta. Toxicol. 1:159–168 (2005).
S. Willmann, J. Lippert, M. Sevestre, J. Solodenko, F. Fois, and W. Schmitt. PK-Sim®: a physiologically based pharmacokinetic ‘whole-body’ model. Biosilico. 1:121–124 (2003).
T. Sawamoto, S. Haruta, Y. Kurosaki, K. Higaki, and T. Kimura. Prediction of the plasma concentration profiles of orally administered drugs in rats on the basis of gastrointestinal transit kinetics and absorbability. J. Pharm. Pharmacol. 49:450–457 (1997).
D. E. Leahy. Intrinsic molecular volume as a measure of the cavity term in linear solvation energy relationships: octanol-water partition coefficients and aqueous solubilities. J. Pharm. Sci. 75:629–636 (1986).
A. Loidl-Stahlhofen, A. Eckert, T. Hartmann, and M. Schottner. Solid-supported lipid membranes as a tool for determination of membrane affinity: high-throughput screening of a physicochemical parameter. J. Pharm. Sci. 90:599–606 (2001).
A. Loidl-Stahlhofen, T. Hartmann, M. Schottner, C. Rohring, H. Brodowsky, J. Schmitt, et al. Multilamellar liposomes and solid-supported lipid membranes (TRANSIL): screening of lipid-water partitioning toward a high-throughput scale. Pharm. Res. 18:1782–1788 (2001).
F. A. Gobas, J. M. Lahittete, G. Garofalo, W. Y. Shiu, and D. Mackay. A novel method for measuring membrane-water partition coefficients of hydrophobic organic chemicals: comparison with 1-octanol-water partitioning. J. Pharm. Sci. 77:265–272 (1988).
T. Makita, T. Anjiki, H. Goto, K. Hakoi, K. Hirabara, T. Ishida, et al. Body and organ weights and the length of intestine of japanese monkey (Macaca Fuscata) II. Yamaguchi J. Vet. Med. 12:97–100 (1985).
Reynolds DG, Brim J, and Sheehy TW. The vascular architecture of the small intestinal mucosa of the monkey (Macaca mulatta). Anat. Rec. 159:211–218 (1967).
D. T. Moran and J. C. Rowley III. Visual Histology. [cited Jun 10, 2006], Available from: http://www.visualhistology.com.
G. M. Grass, and S. A. Sweetana. A correlation of permeabilities for passively transported compounds in monkey and rabbit jejunum. Pharm. Res. 6:857–862 (1989).
ICRP. Basic Anatomical and Physiological Data for Use in Radiological Protection: Reference Values. Elsevier Science Amsterdam, The Netherlands, 2002.
H. Kondo, T. Shinoda, H. Nakashima, T. Watanabe, and S. Yokohama. Characteristics of the gastric pH profiles of unfed and fed cynomolgus monkeys as pharmaceutical product development subjects. Biopharm. Drug Dispos. 24:45–51 (2003).
H. Kondo, T. Watanabe, S. Yokohama, and J. Watanabe. Effect of food on gastrointestinal transit of liquids in cynomolgus monkeys. Biopharm. Drug Dispos. 24:141–151 (2003).
L. Kalantzi, K. Goumas, V. Kalioras, B. Abrahamsson, J. B. Dressman, and C. Reppas. Characterization of the human upper gastrointestinal contents under conditions simulating bioavailability/bioequivalence studies. Pharm. Res. 23:165–176 (2006).
H. M. Siefert, D. Maruhn, W. Maul, D. Forster, and W. Ritter. Pharmacokinetics of ciprofloxacin. 1st communication: absorption, concentrations in plasma, metabolism and excretion after a single administration of [14C]ciprofloxacin in albino rats and rhesus monkeys. Arzneimittelforschung. 36:1496–1502 (1986).
M. Hu, and G. L. Amidon. Passive and carrier-mediated intestinal absorption components of captopril. J. Pharm. Sci. 77:1007–1011 (1988).
P. J. Sinko, and P. V. Balimane. Carrier-mediated intestinal absorption of valacyclovir, the L-valyl ester prodrug of acyclovir: 1. Interactions with peptides, organic anions and organic cations in rats. Biopharm. Drug Dispos. 19:209–217 (1998).
H. Han, R. L. de Vrueh, J. K. Rhie, K. M. Covitz, P. L. Smith, C. P. Lee, et al. 5′-Amino acid esters of antiviral nucleosides, acyclovir, and AZT are absorbed by the intestinal PEPT1 peptide transporter. Pharm. Res. 15:1154–1159 (1998).
S. Vautier, L. Lacomblez, H. Chacun, V. Picard, F. Gimenez, R. Farinotti, et al. Interactions between the dopamine agonist, bromocriptine and the efflux protein, P-glycoprotein at the blood-brain barrier in the mouse. Eur. J. Pharm. Sci. 27:167–174 (2006).
D. S. Wishart, C. Knox, A. C. Guo, S. Shrivastava, M. Hassanali, P. Stothard, et al. DrugBank: a comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 34:D668–D672 (2006).
S. Rendic. Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab. Rev. 34:83–448 (2002).
K. Obata, K. Sugano, R. Saitoh, A. Higashida, Y. Nabuchi, M. Machida, et al. Prediction of oral drug absorption in humans by theoretical passive absorption model. Int. J. Pharm. 293:183–192 (2005).
Y. L. He, S. Murby, L. Gifford, A. Collett, G. Warhurst, K. T. Douglas, et al. Oral absorption of D-oligopeptides in rats via the paracellular route. Pharm. Res. 13:1673–1678 (1996).
J. H. Lin. Bisphosphonates: a review of their pharmacokinetic properties. Bone. 18:75–85 (1996).
M. S. Barnette, C. D. Manning, W. J. Price, and F. C. Barone. Initial biochemical and functional characterization of cyclic nucleotide phosphodiesterase isozymes in canine colonic smooth muscle. J. Pharmacol. Exp. Ther. 264:801–812 (1993).
T. Prueksaritanont, L. M. Gorham, J. H. Hochman, L. O. Tran, and K. P. Vyas. Comparative studies of drug-metabolizing enzymes in dog, monkey, and human small intestines, and in Caco-2 cells. Drug Metab. Dispos. 24:634–642 (1996).
K. W. Ward, G. J. Stelman, J. A. Morgan, K. S. Zeigler, L. M. Azzarano, J. R. Kehler, et al. Development of an in vivo preclinical screen model to estimate absorption and first-pass hepatic extraction of xenobiotics. II. Use of ketoconazole to identify P-glycoprotein/CYP3A-limited bioavailability in the monkey. Drug Metab. Dispos. 32:172–177 (2004).
NIAID. Chemical/Therapeutics Classes. National Institute of Allergy and Infectious Diseases, National Institute of Health, USA [cited Mar 6, 2006], Available from: http://chemdb.niaid.nih.gov/struct_search/class_search.asp.
T. S. Wiedmann, R. Bhatia, and L. W. Wattenberg. Drug solubilization in lung surfactant. J. Control. Release 65:43–47 (2000).
A. Dobashi. 3DPSD. Department of Pharmaceutical Information Science, School of Pharmacy, Tokyo University of Pharmacy and Life Science. [cited Mar 6, 2006]; Available from: http://www.pharmis.org.
S. Ruggieri, F. Stocchi, A. Carta, D. Bravi, M. Bragoni, L. Giorgi, et al. Comparison between L-dopa and lisuride intravenous infusions: a clinical study. Mov. Disord. 3:313–319 (1988).
NCI. National Cancer Institute of Frederick, National Institute of Health, MD, USA. [cited Mar 6, 2006], Available from: http://dtpws4.ncifcrf.gov/DATA/PHARM_DATA/269148.HTML.
European Medicines Agency. European Public Assessment Report: Scientific Discussion, Irbesartan. [cited Mar 6, 2006], Available from: http://www.emea.eu int/humandocs/Humans/EPAR/karvea/karvea.htm.
Sigma-Aldrich. R6520 Rolipram. [Cited Mar 6, 2006], Available from: http://www.sigmaaldrich.com.
Food & Drug Administration. Valtrex Product Information 2001, USA, [Cited Mar 6, 2006], Available from: http://www.fda.gov/cder/foi/label/2001/20550s12lbl.pdf.
E. Deretey, M. Feher, and J. M. Schmidt. Rapid prediction of human intestinal absorption. Quant. Struct.-Act. Relatsh. 21:493–506 (2002).
TerraBase Inc. TerraQSAR-LOGP™ computed octanol/water partition coefficients (CLOGPs). [Cited Mar 6, 2006], Available from: http://www.terrabase-inc.com/anti-inflamms.html.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Willmann, S., Edginton, A.N. & Dressman, J.B. Development and Validation of a Physiology-based Model for the Prediction of Oral Absorption in Monkeys. Pharm Res 24, 1275–1282 (2007). https://doi.org/10.1007/s11095-007-9247-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9247-y