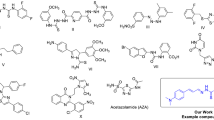
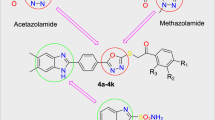
Thirteen novel compounds in a series of dihydrobenzo[h]quinazolin-2-yl thiourea compounds (7a–m) were synthesized and characterized using FT-IR, 1H, 13C NMR spectroscopy, and elemental analysis. Some inhibition parameters including IC50 and inhibition constant values (Ki) were determined for all the compounds. All studied compounds exhibited potent inhibition against carbonic anhydrases (CAs). They inhibited CAs with the IC50 values of 30.45 to 94.00 μM (Ki: 28.27–61.01 μM) for hCA I and 21.80 to 78.00 μM (Ki: 17.84–57.96 μM) for hCA II. The most active compounds were found to be compound 7m for hCA I (Ki: 28.27 μM) and compound 7d for hCA II (Ki : 17.84 μM). The absorption, distribution, metabolism, excretion, and toxicity (ADME-Tox) study revealed that all the derivatives had good oral bioavailability with respect to Lipinski’s rule of five and Jorgensen’s rule of three. All derivatives in the series can be considered as outstanding multitarget inhibitors for further investigations.
Similar content being viewed by others
References
R. Dua, S. Shrivastava, S. K. Sonwane, et al., Adv. Biol. Res., 5, 120 – 144 (2011).
A. Gomtsyan, Chem. Heterocycl. Compd., 48, 7 – 10 (2012).
M. Koos, Chem. Pap., 48, 108 – 110 (1994).
F. R. Alexandre, A. Berecibar, R.Wrigglesworth, et al., Tetrahedron, 59, 1413 (2013).
D. J. Connolly, D. Cusack, T. P. O’Sullivan, et al., Tetrahedron, 61, 10153 (2005).
P. Nayyar, R. Arpana, I. Mohd, et al., Int. J. Pharm. Biol. Sci. Arch., 2, 1651 – 1657 (2011).
C. S. Chu, G. Bancone, F. Nosten, et al., Malar. J., 17, 1 – 9 (2018).
N. M. Heron, M. Anderson, D. P. Blowers, et al., Bioorg. Med. Chem. Lett., 16, 1320 (2006).
L. Zhu, J. Jin, C. Liu, et.al., Bioorg. Med. Chem. Lett., 19, 2797 – 2807 (2011).
K. C. Agarwal, V. Sharma, N. Shakya, et.al., Bioorg. Med. Chem. Lett., 19, 5474 (2009).
S. Sasmal, D. Balasubrahmanyam, H. R. Kanna Reddy, et al., Bioorg. Med. Chem. Lett., 22, 3163 – 3167 (2012).
C. S. Shantharam, V. M. Suyoga, R. Suhas, et al., Eur. J. Med. Chem., 60, 325 (2013)
A. Claude, K. N. Devi, S. Karthick, et al., Int. J. Chem. Tech. Res., 5, 512 (2013).
E. Khan, S. Khan, Z. Gul, et.al., Crit. Rev. Anal. Chem., 51, 812 (2020).
R. Pingaew, V. Prachayasittikul, N. Anuwongcharoen, et al., Bioorg. Chem., 79, 171 – 178 (2018).
A. Celen, B. Kaymakcioglu, S. Gümrü, et al., Marmara Pharm. J., 15, 43 – 47 (2011).
R. S. Keri, M. R. Patil, S. A. Patil, et al., Eur. J. Med. Chem., 89, 207 – 251 (2015).
A. Maalik, H. Rahim, M. Saleem, et al., Bioorg. Chem., 88, 10294 (2019).
S. Prachayasittikul, R. Pingaew, A. Worachartcheewan, et al., Mini. Rev. Med. Chem., 17, 869 – 901 (2017).
A. Mishra and S. Batra, Curr. Top. Med. Chem., 13, 2011 – 2025 (2013).
S. Saeed, N. Rashid, P. G. Jones, et al., Eur. J. Med. Chem., 45, 200 – 205 (2010).
A. Irfan, F. Batool, S. Naqvi, et al., J. Enzyme. Inhib. Med. Chem., 35, 265 – 279 (2020).
S. A. Zimmerman, J. G. Ferry, C. T. Supuran, Curr. Top. Med. Chem., 7, 901 (2007).
C. T. Supuran and A. Scozzafava, Bioorg. Med. Chem., 15, 4336 – 4350 (2007).
C. T. Supuran, Nat. Rev. Drug. Discov., 7, 168 – 181 (2008).
F. Carta, M. Aggarwal, A. Maresca, et al., J. Med. Chem., 55, 1721 – 1730 (2012).
A. R. Nixha, A. Ergun, N. Gencer, et al., Arch. Physiol. Biochem., 125, 263 (2019).
F. Celik , M. Arslan, E. Yavuz, et al., J. Enzyme. Inhib. Med. Chem., 29, 18 – 22 (2014).
A. R. Nixha, M. Arslan, Y. Atalay, et al., J. Enzyme. Inhib. Med. Chem., 28, 808 – 815 (2012).
N. Berber, M. Arslan, E. Yavuz, et al., J. Chem., 8, 742178 (2013).
M. Bozdag, S. Isik, S. Beyaztas, et al., J. Enzyme. Inhib. Med. Chem., 30, 240 – 244 (2015).
J. A. Verpoorte, S. Mehta, J. T. Edsall, J. Biol. Chem., 242, 4221 – 4229 (1967).
H. Lineweaver and D. Burk, J. Am. Chem. Soc., 56, 658 – 666 (1934).
T. Kennedy, Drug. Discov. Today, 2, 436 – 444 (1997).
M. Kalaycı, C. Türkeş, M. Arslan, et al., Arch. Pharm., 354, 200 – 282 (2021).
S. Gündoğdu, C. Türkeş, M. Arslan, et al., ChemistrySelect., 4, 13347 – 13355 (2019).
Q. Istrefi, C. Türkeş, M. Arslan, et al., Arch. Pharm., 353, 1900383 (2020).
C. Turkes, M. Arslan, Y. Demir, et al., Bioorg. Chem., 89, 103004 (2019).
C. A. Lipinski, F. Lombardo, B. W. Dominy, et al., Adv. Drug. Deliv. Rev., 23, 3 – 25 (1997).
E. M. Duffy andW. L. Jorgensen, J. Am. Chem. Soc., 122, 2878 – 2888 (2000).
C. T. Supuran and A. Scozzafava, Expert. Opin. Ther. Pat., 10, 575 – 600 (2000).
H. Ogita, Y. Isobe, H. Takaku, et al., Bioorg. Med. Chem., 10, 1865 – 1871 (2002).
O. Arslan, O. I. Kufrevioglu, B. Nalbantoglu, Bioorg. Med. Chem., 5, 515 – 518 (1997).
S. Qaiser,M. S. Mubarak, S. Ashraf, et al., Med. Chem. Res., 30, 552 – 563 (2021).
M. Tugrak, H. I. Gul, Y. Demir, et al., Arch. Pharm., 354, 200 – 230 (2021).
B. Z. Kurt, F. Sonmez, S. Durdagi, et al., J. Enzyme. Inhib. Med. Chem., 32, 1042 – 1052 (2017).
J. Moeker, K. Teruya, S. Rossit, et al., Bioorg. Med. Chem., 20, 2392 (2012).
E. Domínguez-Álvarez, D. Łażewska, Z. Szabó, et al., ChemistrySelect., 4, 10943 – 10952 (2019).
V. Ravichandran, S. Shalini, K. S. Kumar, et al., Lett. Drug. Des. Discov., 16, 618 (2016).
M. Mahdavi, M. S. Shirazi, R. Taherkhani, et.al., Eur. J. Med. Chem., 82, 308 – 313 (2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Taşcı, M., Arslan, M., Çıkrıkcı, K. et al. Synthesis and Investigation of Carbonic Anhydrase I and II Activity of Dihydrobenzo[h]Quinazolin-2-yl Thiourea Compounds. Pharm Chem J 57, 899–906 (2023). https://doi.org/10.1007/s11094-023-02965-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02965-3