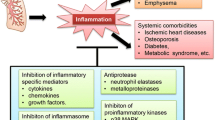
We have studied the clinical efficacy of long-acting β2-agonist (LABA) and long-acting muscarinic antagonist (LAMA) for the treatment of moderate to severe chronic obstructive pulmonary disease (COPD). For this purpose, clinical data of 145 patients with moderate to severe COPD diagnoses were gained in our hospital and retrospective analyzed. Patients were assigned into a research group (49 patients) and two control (LABA and LAMA) groups (48 patients each). Patients in the LABA, LAMA and research groups were given indacaterol maleate powder (trade name Onbrez) for inhalation, tiotropium bromide powder (trade name Tianqingsule) for inhalation and a combination of these drugs, respectively. After treatment, the efficacy, as well as the improvement of the pulmonary function, vascular endothelial function, motor function and inflammatory factors were compared among the three group. The total effective rate of treatment in the research group was significantly higher than that in the two control groups, while the number of rehospitalization cases showed an opposite trend. After treatment, the pulmonary function, respiratory muscle strength, and 6-minute walk distance in the research group were significantly higher than those in the two control groups, while the scores of dyspnea showed opposite trends. The improvement in pulmonary function in the LAMA group was also significantly higher than that in the LABA group. In addition, the post- treatment values of the von Willebrand factor, endothelium-1, interleukin (IL)-6, IL-8 and tumor necrosis factor (TNF)-α levels were significantly lower in the research group than those in the two control groups, while the vascular endothelial growth factor and nitric oxide levels were significantly higher. Besides, there was no significant difference in the incidence of adverse reactions among the three groups. Furthermore, there were no significant differences in the total effective rate of treatment, the number of rehospitalizations, respiratory muscle strength, dyspnea score, 6-minute walk distance, vascular endothelial function and inflammatory factors between the two control groups. It was concluded that the combination of LABA and LAMA was more effective than either drug alone for treating moderate to severe COPD, showing more significant effects on the improvement of pulmonary function, vascular endothelial function and dyspnea symptoms, without increasing adverse effects and higher safety.


Similar content being viewed by others
References
M. B. Stanbrook, Ann. Intern. Med., 169, 112 – 114 (2018).
K. Gorska, P. Nejman-Gryz, M. Paplinska-Goryca, et al., COPD, 15, 36 – 45 (2018).
J. A. Wedzicha, D. Singh, I. Tsiligianni, et al., Respir. Res., 20, 4 (2019).
S. Suiss., S. Dell’Aniello, P. Ernst, Chest, 155, 1158 – 1165 (2019).
C. Lyon, R. Radi, T. Staff, et al., J. Fam. Pract., 67, 384 – 385 (2018).
S. Yang, Y. Zheng, X. Hou, Cell Signal., 60, 146 – 153 (2019).
D. Stolz, K. Kostikas, E. Loefroth, et al., Chest, 156, 674 – 684 (2019).
A. F. Chi, A. Ctan, O. Soriu, et al., Eur. J. Inflamm., 18, 205873922096646 (2020).
F. J. Martinez, K. F. Rabe, G. T. Ferguson, et al., Am. J. Respir. Crit. Care Med., 203, 553 – 564 (2021).
M. Mantero, D. Radovanovic, P. Santus, et al., Int. J. Chron. Obstruct. Pulmon. Dis., 13, 2319 – 2333 (2018).
M. K. Han, R. Ray, J. Foo, et al., NPJ Prim. Care Respir. Med., 28, 32 (2018).
H. Demeyer, M. Costilla-Frias, Z. Louvaris, et al., Eur. Respir. J., 51, 1174 – 1178 (2018).
G. A. Heresi, Y. Rao, Lung, 198, 933 – 938 (2020).
J. Luo, Y. H. Liu, W. Luo, et al., Sci. Rep., 9, 4109 (2019).
N. Stakenborg, E. Labeeuw, P. J. Gomez-Pinilla, et al., Gut, 68, 1406 – 1416 (2019).
B. Z. Shao, S. L. Wang, J. Fang, et al., Inflammation, 42, 1666 – 1679 (2019).
R. A. Sitapara, A. G. Gauthier, S. I. Valdés-Ferrer, et al., Mol. Med., 26, 63 (2020).
D. Bagdas, D. Ergun, A. Jackson, et al., Eur. J. Pain, 22, 84 – 93 (2018).
M. Azimi, M. Oemisch, T. Womelsdorf, Psychopharmacology (Berl.), 237, 997 – 1010 (2020).
M. R. Farlow, S. M. Graham, G. Alva, Drug Saf., 31, 577 – 585 (2008).
A. P. Milstone, Int. J. Chron. Obstruct. Pulmon. Dis., 3, 515 – 520 (2008).
J. Y. So, S. Dhungana, J. J. Beros, et al., Curr. Opin. Pharmacol., 40, 26 – 33 (2018).
T. Kamei, H. D. Nakamura, N. D. Nanki, et al., BMJ Open, 9, e024114 (2019).
X. F. Xiong, L. L. Fan, H. X. Wu, et al., Adv. Ther., 35, 2201 – 2213 (2018).
D. Singh, A. Ravi, K. Kane, et al., Br. J. Clin. Pharmacol., 84, 2097 – 2105 (2018).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Y., Pang, Y. Clinical Efficacy of Long-Acting β2 Agonists and Long-Acting Muscarinic Antagonist for Moderate to Severe Chronic Obstructive Pulmonary Disease. Pharm Chem J 57, 365–372 (2023). https://doi.org/10.1007/s11094-023-02891-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-023-02891-4