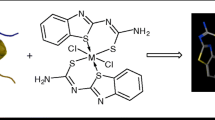
Biological activities of organoruthenium complexes [chloro [N,N′-[(2,6-pyridinediyl-κN) diethylidyne] bis-[benzenamine-κN]] [N-[(2-pyridinyl-κN) methylene] benzenesulfonamide-κN] ruthenium(II)] chloride (Cmplx 1), [chloro [2,2′-(2,6-pyridinediyl-κN) bis [1H-benzimidazole-κN3]][N-[(2-pyridinyl-κN) methylene] benzenesulfonamide-κN] ruthenium(II)] chloride (Cmplx 2), and [chloro[2,6-di(1H-pyrazol-3-yl-κN2) pyridine-κN] [N-[(2-pyridinyl-κN) methylene] [benzenesulfonamide-κN] ruthenium(II)] chloride (Cmplx 3) have been studies. The compounds were tested for in vitro biological activity on test models including 2,2-diphenyl-1-picryl-hydrazyl (DPPH) reducing power, superoxide anion radical-scavenging activity, and lipid peroxidation activity by ferric thiocyanate. It is established that Cmplx 2 with benzimidazole ligand displays significant xanthine oxidase inhibitory activity (IC50 = 53.80 ± 2.69 μM), DPPH free radical scavenging activity (79.49 ± 1.59), and superoxide anion radical scavenging activity (75.73 ± 2.85%). The coordination of benzenamine and benzenesulfanoamine ligands reduces lipid peroxidation as observed in the case of Cmplx 1 (87.17 ± 3.88%) and the higher reducing power of Cmplx 1 obtained at all concentrations. It was concluded from the test results that organoruthenium complexes showed much better antioxidant activity than expected.






Similar content being viewed by others
References
C. Sun, J. Wang, L. Fang, et al., Life Sci., 75, 1063 – 1073 (2004).
H. Haraguchi, Antioxidative Plant Constituents, in: C. Tingali (Ed.), Bioactive Compounds from Natural Sources, New York: Taylor and Francis (2001), pp. 338 – 377
O. I. Aruoma, J. Am. Oil. Chem. Soc.,73, 1617 – 1625 (1996).
T. Akaike, M. Ando, T. Oda, T et al., J. Clin. Invest.,85, 739 – 745 (1990).
M. L. Ferrandiz and M. J. Alcaraz, Agents Actions, 32, 283 – 288 (1991).
E. Kokoglu, A. Belce, E. Ozyurt, and Z. Tepeler, Cancer Lett., 50 (1990) 179 – 181.
J. M. McCord, New Engl. J. Med.,312, 159 – 163 (1985).
T. Nishino S. Nakanishi, K. Okamoto, et al., Biochem Soc Trans., 25, 783 – 786 (1997).
H. Tsutomu, Y. Taeko, Y. Rieko, et al., Planta Med., 57, 83 – 84 (1991).
C. G. Hartinger, A. Casini, C. Dyhot, et al., J. Inorg. Biochem., 12, 2136 – 2141 (2008).
E. Alessio, Chem. Rev., 104, 4203 – 4242 (2004).
F. Wang, J. A. Xu, A. Habtemariam, et al., J. Am. Chem. Soc., 127, 17734 – 17743 (2005).
D. Griffith, S. Cecco, E. Zagrando, et al., J. Biol. Inorg. Chem., 13, 511 – 520 (2008).
V. B. Arion, E. Reisner, M. Fremuth, et al., Inorg. Chem., 42, 6024 – 6031 (2003).
W. Kandioler, C. G. Hartinger, A. A. Nazarov, et al., J. Org. Chem., 694, 922 – 929 (2009).
P. J. Dyson and G. Sava, Dalton Trans., 16, 1929 – 1933 (2006).
C. A. Vock, W. H. Ang, C. Scolaro, et al., J. Med. Chem., 50, 2166 – 2175 (2007).
A. Dorcier, C. G. Hartinger, R. Scopelliti, et al., J. Inorg Biochem., 102, 1066 – 1076 (2008).
M. J. Clarke, F. Zhu, and D. R. Frasca, Chem. Rev., 99, 2511 – 2533 (1999).
I. Bratsos, B. Serli, E. Zagrando, et al,., Inorg. Chem., 46, 975 – 992 (2007).
T. W. Hambley, Dalton Trans., 43, 4929 – 4937 (2007).
C. S. Allardyce, A. Dorcier, C. Scolaro, and P. J. Dyson, Appl. Organomet. Chem., 19, 1 – 10 (2005).
C. X. Zhang and S. J. Lippard, Curr. Opin. Chem. Biol., 7, 481 – 489 (2003).
S. Kapitza, M. Pongratz, M. A. Jakupec, et al., J. Cancer Clin. Oncol., 131, 101 – 120 (2005).
H. J. Park, K. Lee, S. Park, et al., Bioorg. Med. Chem. Lett., 15, 3307 – 3312 (2005).
I. Bouabdallah, L. A. M’barek, A. Zyad, et al., Nat. Prod. Res., 20, 1024 – 1030 (2006).
Y. L. Hong, P. A. Hossler, D. H. Calhoun, and S. R. Meshnic, Antimicrob. Agents Chemother., 39, 1756 – 1763 (1995).
M. N. L. Nalam, A. Peeters, T. H. M. Jonckers, et al., J. Virol., 81, 9512 – 9518 (2007).
M. A. Babizhayev, Life Sci., 78, 2343 – 2357 (2006).
G. Roman, J. G. Riley, J. Z. Vlahakis, et al., Bioorg. Med. Chem., 15, 3225 – 3234 (2007).
N. B. Patel, S. N. Agravat, and F. M. Shaikh, Med. Chem. Res., 20, 1033 – 1041 (2011).
N. B. Patel and S. N. Agravat, Chem. Heterocycl. Compds., 45, 1343 – 1353 (2009).
S. Gulnaz, N. Özdemir, S. Dayan, et al., Organometallics, 30, 4165 – 4173 (2011).
L. Marcocci, L. Packer, M. T. Droy-Lefaix, et al., in: L. Parker (Ed.), Methods in Enzymology, San Diego: Academic Press (1994), pp. 462 – 475.
T. Osawa and M. Namiki, Agric. Biol. Chem., 45, 735 – 740 (1981).
F. Liu, V. E. C. Ooi, and S. T. Chang, Life Sci., 60, 763 (1997).
A. Ardestani and R. Yazdanparast, Food Chem., 104, 21 – 29 (2007).
M. Oyaizu, Jpn. J. Nutr., 103, 413 – 419 (1986).
Acknowledgements
The authors thank for financial support the Scientific and Technological Research Council of Turkey (TÜBÝTAK; 111T560) and the Scientific Research Project Department of Bingol University (BÜBAP; 2010 – 07)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gecibesler, Ý.H., Dayan, O., Şerbetçi, Z. et al. Antioxidant Activity of Ruthenium(Ii) Complexes Containing Tridentate Triamines and Their Capability to Inhibit Xanthine Oxidase. Pharm Chem J 53, 914–920 (2020). https://doi.org/10.1007/s11094-020-02099-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-020-02099-w