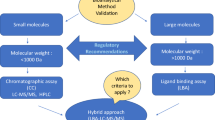
US FDA requirements published in the new 2018 guidance for bioanalytical method validation and the necessity to confirm their reliability for determining analyte concentrations are reviewed. The history of regulations for bioanalytical method validation is briefly described. The key changes and additions to the FDA guidance for bioanalytical method validation that include unified requirements for chromatographic and ligand-binding method validation, instructions for biomarker validation, a separate section focused on new technologies (e.g., dry blood-spot method), and introduction of the fit-for-purpose concept are discussed. FDA and EAEU requirements for validation of chromatographic assay parameters are compared. In general, the requirements of the new FDA guidance and the EAEU agree despite several differences in the number of parameters and their acceptance criteria.
Similar content being viewed by others
References
V. V. Chistyakov, Vedom. NTsESMP, No. 3, 24 – 28 (2013).
Guidance for Industry: Bioanalytical Method Validation, U. S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CMV), 2018.
Guideline on bioanalytical method validation (EMEA/CHMP/ EWP/192217/2009 Rev. 1 Corr. 2), European Medicines Agency, London, 2011.
V. P. Shah, K. K. Midha, S. V. Dighe, et al., Pharm. Res., 9, 588 – 592 (1992).
G. V. Ramenskaya, I. E. Shokhin, A. Yu. Savchenko, et al., Remedium, No. 12, 60 – 63 (2011).
A. N. Mironov (ed.), Handbook for Drug Review [in Russian], Vol. 1, Grif i K, Moscow, 2013, pp. 201 – 215.
Rules for Drug Bioequivalence Studies in the Eurasian Economic Union Approved by Decision No. 85 of the Eurasian Economic Commission Council Dated Nov. 3, 2016, Moscow, 2016.
L. V. Sonawane, B. N. Poul, S. V. Usnale, et al., Pharm. Anal. Acta, 5(3), 1 – 7 (2014).
Guidance for Industry: Bioanalytical Method Validation, U. S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CMV), 2001.
V. P. Shah, AAPS J., 9(1), E43-E47 (2007).
R. J. Meesters and S. Voswinkel, J. Appl. Bioanal., 4(3), 67 – 73 (2018).
N. Kadian, K. S. Raju, M. Rashid, et al., J. Pharm. Biomed. Anal., 126, 83 – 97 (2016).
Guidance for Industry: Bioanalytical Method Validation (Draft Guidance), U. S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CMV), 2013.
D. Zimmer, Bioanalysis, 6(1), 13 – 19 (2014).
2018 Bioanalytical Method Validation Guidance: Key Changes and Consideration; (on-line) https: //www.q2labsolutions.com/blogs/.
R. K. Srivastava and S. S. Kumar, Eur. J. Pharm. Med. Res., 4(09), 774 – 784 (2017).
Acknowledgments
The work was sponsored by a state task to SCEEMP, Ministry of Health of Russia, No. 056-0154-9-0 for applied scientific research (State Account No. NIR AAAA-A18-18021590049-).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 53, No. 8, pp. 45 – 52, August, 2019.
Rights and permissions
About this article
Cite this article
Uvarova, N.E., Eremenko, N.N., Ramenskaya, G.V. et al. Comparison of FDA (2018) and EAEU Regulatory Requirements for Bioanalytical Method Validation. Pharm Chem J 53, 759–765 (2019). https://doi.org/10.1007/s11094-019-02075-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-019-02075-z