Abstract
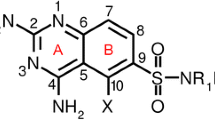
Quantitative structure-pharmacokinetic/pharmacodynamic (PK/PD) relationship (QSPR) techniques and chemometric methods were employed to classify fluoroquinolones with respect to their activity against Streptococcus pneumoniae. Density functional theory (DFT) was used to calculate a set of molecular descriptors (properties) for 13 synthetic fluoroquinolones. The descriptors were further analyzed using chemometric methods including principal component analysis (PCA), hierarchical cluster analysis (HCA), and stepwise discriminant analysis (SDA). The PCA and SDA methods were employed in order to reduce the dimensionality and select a subset of variables that would be more effective for classifying the fluoroquinolones according to their degree of antipneumococcal activity. The methods of PCA, SDA and HCA were quite efficient to classify 13 compounds in two groups (active and inactive), and the net charge on ring B (Q B), molecular volume (VOL), and partition coefficient (log P ) were found to be descriptors important for the classification. These methodologies of PCA, SDA and HCA provide a reliable rule for classifying new fluoroquinolones with respect to antipneumococcal activity. The application of the SPP relationship is of considerable value for clinicians, drug developers, and regulators because PK/PD principles form the basis of modern antimicrobial chemotherapy.
Similar content being viewed by others
References
M. R. Jacobs, Int. J. Infect Dis., No. 7, 13–20 (2003).
W. A. Craig, Clin. Infect. Dis., 26, 1–12 (1997).
W. A. Craig, Pharmacodynamics of Antimicrobials: General Concepts and Application, in Antimicrobial Pharmacodynamics in Theory and Clinical Practice, C. H. Nightingale, T. Murakawa, P. G. Ambrose (eds.), Marcel Dekker, New York (2002), pp. 1–21.
M. J. Frisch, G. W. Truck, H. B. Schlegel, et al., GAUSSIAN 03 (Revision 03), Gaussian Inc., Pittsburgh PA (2003).
R. G. Parr and W. Yang, Density-Functional Theory of Atoms and Molecules, Oxford-New York (1989).
A. D. Becke, Phys. Rev. A, 38, 3098–3100 (1988).
A. D. J. Becke, Chem. Phys., 98, 5648–5652 (1993).
W. Khon, A. D. Becke, and R. G. J. Parr, J. Phys. Chem., 100, 12974–12980 (1996).
G. G. Zhanel, K. Ennis, L. Vercaigne, et al., Drugs, 62, 13–59 (2002).
G. G. Zhanel, J. A. Karlowsky, L. Palatnick, et al., Antimicrob. Agents Chemother., 43, 2504–2509 (1999).
G. G. Zhanel, Curr. Infect. Dis. Rep., 3, 29–34 (2001).
G. G. Zhanel, M. Walters, D. Roberts, et al., J. Antimicrob. Chemother., 47, 435–440 (2001).
G. L. Ridgway, M. D. O'Hare, D. Felmingham, and R. N. Grüneberg, Drugs Exp. Clin. Res., 11, 259–262 (1985).
A. Bauernfeind, J. Antimicrob. Chemother., 40, 639–651 (1997).
D. Felmingham, M. J. Robbins, A. Leakey, et al., In vitro Activity of Moxifloxacin, in Moxifloxacin in Practice, D. Adam, R. Finch R, and P. Hunter (eds.), Maxim Medical, Oxford (1999), Vol. 2, pp. 27–37.
D. B. Hoellman, G. Lin, M. R. Jacobs, and P. C. Appelbaum, J. Antimicrob. Chemother., 43, 45–649 (1999).
M. A. Visalli, M. R. Jacobs, and P. C. Appelbaum, Antimicrob. Agents. Chemother., 41, 2786–2789 (1997).
L. M. Ednie, M. R. Jacobs, and P. C. Appelbaum, Antimicrob. Agents Chemother., 42, 1269–1273 (1997).
T. Nakane, S. Iyobe, K. Sato, and S. Mitsuhashi, Antimicrob. Agents Chemother., 39, 2822–2826 (1995).
M. G. Cormican, and R. N. Jones, Antimicrob. Agents Chemother., 41, 204–211 (1997).
J. M. Woodcock, J. M. Andrews, J. F. Boswell, et al., Antimicrob. Agents Chemother, 41, 101–106 (1997).
J. J. Schentag, K. K. Gilliland, J. A. Paladino, Clin. Infect. Dis., 32(suppl), 39–46 (2001).
E. J. Dolestein and S. M. Garabedian-Ruffalo, Clin. Infect. Dis., 35, 1505–1511 (2002).
H. V. Waterbeemd, N. El Tayar, P.-A. Carrupt, and B. Testa, J. Comput.-Aided Mol. Design., 3, 111–132 (1989).
R. A. Johnson and D. W. Wichern, Applied Multivariate Statistical Analysis, Prentice-Hall, Englewood Cliffs, NJ (1992).
K. V. Mardia, J. T. Kent, and J. M. Bibbly, Multivariate Analysis, Academic Press, New York (1979).
Author information
Authors and Affiliations
Additional information
__________
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 41, No. 2, pp. 23–28, February, 2007.
Rights and permissions
About this article
Cite this article
Li, XH., Zhu, ZL., Cheng, XL. et al. Quantitative structure-pharmacokinetic/pharmacodynamic relationship for fluoroquinolones. Pharm Chem J 41, 82–87 (2007). https://doi.org/10.1007/s11094-007-0018-1
Issue Date:
DOI: https://doi.org/10.1007/s11094-007-0018-1